|
|
Journal of Advanced Veterinary Research Volume 9, Issue 2, 2019, Pages: 56-63 |
|
|
Ultrasonographic Monitoring of Reproductive Organs of Barki Rams during early Non- Breeding Season |
||||||||||||||||||||
|
|
||||||||||||||||||||
|
Hamed T. Elbaz*, Emad M. Abdel Razek |
||||||||||||||||||||
|
|
||||||||||||||||||||
|
Theriogenology Department, Faculty of Veterinary Medicine, University of Sadat City, Menofia, Egypt. |
||||||||||||||||||||
|
|
||||||||||||||||||||
|
Received: 30 January 2019, Accepted: 26 March 2019 |
||||||||||||||||||||
|
*: Corresponding author: hamedvet2020@yahoo.com |
||||||||||||||||||||
|
|
||||||||||||||||||||
|
Abstract |
||||||||||||||||||||
|
|
||||||||||||||||||||
|
The aim of the present study was to monitor the testes and the accessory sex glands in ram during early non-breeding season with ultrasonography. A clinically healthy eight adult Egyptian Barki rams were used in this study to compare ultrasonographic and measurements of reproductive organs. A scanning technique based on multiple imaging of scrotal contents and also imaging pelvic urethra and accessory sex glands. The results revealed that scrotum circumference was 28.87±1.1 cm and the testicular parenchyma was homogeneous granular medium gray to dark gray. The mediastinum testis appeared as central white linear structure of greater echogenicity than testicular parenchyma. The tail of the epididymis was clearly visible, less echoic than the testicular parenchyma with a more heterogeneous structure. Ampulla appeared as a non-echogenic to hypoechogenic linear lumen. Vesicular gland appeared as a heterogeneous hypo-echogenic structure with irregular outline and circumscribed with echogenic line. The lumen of the pelvic urethra appeared as non-echogenic tube surrounded by moderately echogenic urethral muscle. Pars disseminata of prostate was seen spread along the lumen of the pelvic urethra. Bulbourethral glands appeared with variable echogenicity from hypo-echogenicity to moderate echogenicity. From this study, it could be concluded that the echogenicity and the biometry of accessory sex glands and testes are important parameters for the selection of breeding rams. |
||||||||||||||||||||
|
|
||||||||||||||||||||
|
Keywords: Rams, Testis, Epididymis, accessory sex glands, Ultrasonography |
||||||||||||||||||||
|
|
||||||||||||||||||||
|
Introduction |
||||||||||||||||||||
|
|
||||||||||||||||||||
|
Breeding soundness examination of rams should be done before the beginning of the breeding season to eliminate sub-fertile and evaluate the reproductive ability of rams (Gouletsou et al., 2003; Gouletsou and Fthenakis, 2010). Ultrasound scanning was reported to be an important tool in examination of ram’s genitalia and interpretation of uncertain clinical findings and early lesions (Gouletsou et al., 2003). The use of ultrasound in examination of the testicles in small ruminants has been reported by many studies (Andrade et al., 2014; Gouletsou, 2017; Ribeiro et al., 2017). Ovine male genitalia can be visualized by ultrasonography using a frequency of 5-7.5MHz (Gouletsou et al., 2003; Sargison, 2008). The B-mode, grey-scale ultrasonography enables the visualization of internal reproductive organs (Chandolia et al., 1997). Testicular ultrasound findings may help to assess the reproductive status of breeders and can be used in diagnosis of seminiferous tubule calcification (Ahmadi et al., 2012). Furthermore, ultrasonographic examination of the testicles width can provide an accurate measure of testicle sperm production (Cartee et al., 1990; Kastelic et al., 2001; Najjar et al., 2012). Recent technical advances of U.S. applications and post processing developments have enabled new aspects in the structural and functional analysis of testicular tissue and improving the male fertility (Schurich et al., 2009). The reproductive activity of sheep is influenced by seasonal factors such as temperature, relative humidity and photoperiods (Hulet et al., 1986). Alterations in daylight are major factor causing changes in oestrus activity and male reproduction in subtropical sheep (Aboul-Naga and Aboul-Ela, 1987). Moreover, conception rate in Egyptian sheep rises to maximum in autumn breeding season (Aboul-Naga et al., 1992; Ahmed, 2008). In addition, Perez et al. (1997) found that rams expressed seasonal variation in their reproductive capacities and the testis activity was highest in late summer/early autumn and lowest in winter. There are few papers regarding echogenicity and measurements of vesicular and bulbourethral glands in sheep, which can be used as complementary parameters to assist in the selection of rams with greater sexual activity (Ribeiro et al., 2017). Furthermore, accessory sex glands received very little attention during routine evaluation for breeding soundness of livestock species (Gouletsou and Fthenakis, 2010). The application of trans-rectal ultrasonography studies of sex glands in rams is still rare (Camela et al., 2017). Recently, it was reported that application of contrast-enhanced ultrasound in sheep may have multiple indications, such as differentiation between benign and malignant lesions (Vanderperren et al., 2017). Moreover, variations in testicular lipid content within physiological levels can be estimated using computerized analysis of the testicular ultrasonograms (Ahmadi et al., 2013). The aim of this study was to characterize the size and sonographic features of the ampullae of the ductus deference, vesicular glands, pars disseminata of prostate and bulbourethral glands with evaluation of the scrotum and its contents in the Egyptian Barki rams during the early non-breeding season. In this way, a reference standard would be available in order to image and interpret abnormal features subsequently. |
||||||||||||||||||||
|
|
||||||||||||||||||||
|
Materials and methods |
||||||||||||||||||||
|
Animals The present study was carried out on a total number of eight adult rams (Barki breed) belonged to the educational farm, Faculty of Veterinary Medicine, Sadat City University, Egypt. Rams were aged between 2 and 2.5 years and with body weights ranged from 45 to 55 kg. This study was performed during the period from April to May (2018), which is characterized by long day light and also, the location of farm has a desert climate. All rams were apparently healthy, housed in free stall barn and fed on a balanced ration (14% crude protein, 15% crude fiber, 9% ash and 2% fat), as well as animals had a free access for the drinking water and green fodders (Alfalfa). Testis measurement Scrotal circumference was measured by measuring steel tape (Ahmed and Noakes, 1995). Testis length was measured from top of the tail to the head of the epididymis for each testis using a caliper (Islam and Land, 1977). Ultrasonographic examination of the scrotum and its content Ultrasonographic imaging of the testes and epididymis were done in the standing position. An ultrasound examination of rams was done by means of 5-7.5 MHz linear probe of Scanner (Sonoscape-A5V, Shenzhen, China) to investigate the testis, epididymis and spermatic cord according to the methods that were described previously (Gouletsou et al., 2003; Mahmoud et al., 2013; Camela et al., 2017). Briefly, the animal restrained by an assistant to lift the tail. Scrotal hair was trimmed and shaved for the evaluation of echotexture and echogenicity of the testicular parenchyma. The testes were pulled downwards within the scrotum. The examiner’s left hand was placed on the surface opposite to the one where the transducer with coupling gel is applied upon. The transducer was placed on the caudal surface of the testis along its longitudinal axis (sagittal plane) and moved from right to left. The transducer was moved upwards to image the head of the epididymis and the pampiniform plexus and then the transducer moved downwards to image the tail of epididymis. The procedure was repeated for the other testis. Ultrasonographic examination of the accessory sex glands The transducer was fitted in a self-manufactured connector to favor its manipulation per rectum. The ultrasound transducer was lubricated with coupling gel, placed in the rectum after evacuation of the feces and then was moved cranially to image the urinary bladder-pelvic urethral junction as a guide. The accessory sex glands (ampulla, seminal glands) were visualized near to the urinary bladder. While, pars disseminate of prostate gland was visualized during imaging the pelvic urethra. The bulbourethral glands appeared easily at the exit of rectal probe from the anal opening. The dimensions of the accessory sex glands were measured by ultrasound electronic caliper and echogenicity were evaluated. Cross and longitudinal sections of the pelvic urethra were visualized and examined. Two dimensions (dorso-ventral and cranio-caudal) of vesicular and bulbourethral glands were taken. The prostate gland was measured by dorso-ventral dimension inside the pelvic urethra. All the ultrasound machine settings including the near and far gain and contrast were kept constant throughout the study. This study was assessed and agreed by the Animal Care and Welfare Committee Ethics, Sadat City University, Egypt. Statistical analysis Data are presented as Means ± standard errors of means (SEM). Descriptive statistical analysis was performed using GraphPad prism 5 software Inc., La Jolla, CA. |
||||||||||||||||||||
|
|
||||||||||||||||||||
|
Results |
||||||||||||||||||||
|
Neither affection nor clinical abnormalities were recorded in the genitalia during the clinical examination of rams included in this study. By palpation, the testicles were firm with a homogenous consistency and no pain reaction was recorded. The mean scrotum circumference was 28.87±1.1 cm and the morphometric measurements of the right and left testis lengths were 9.25±0.36 and 9.50±0.37 respectively. Ultrasonographic measurement of scrotum and its contents and accessory sex glands were shown in Table 1. Table 1. Ultrasonographic measurements (mm) of the scrotum and its content, accessory sex glands and scrotum circumference (cm) in Barki rams.
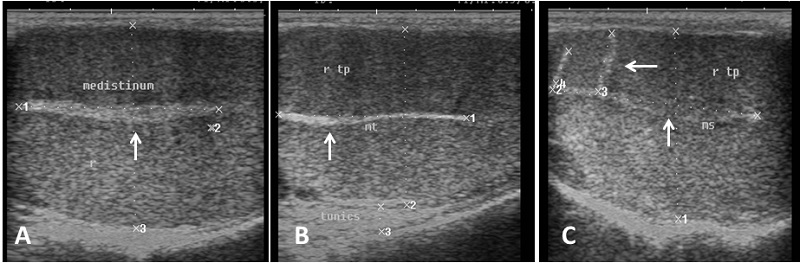
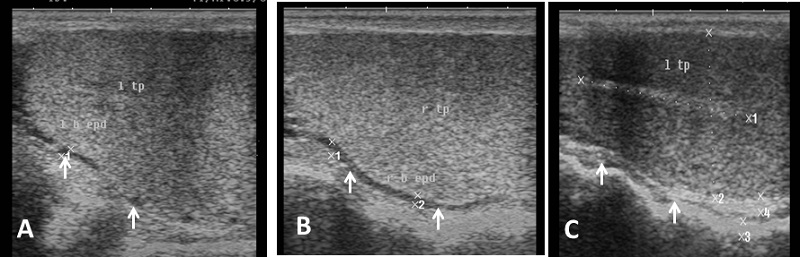
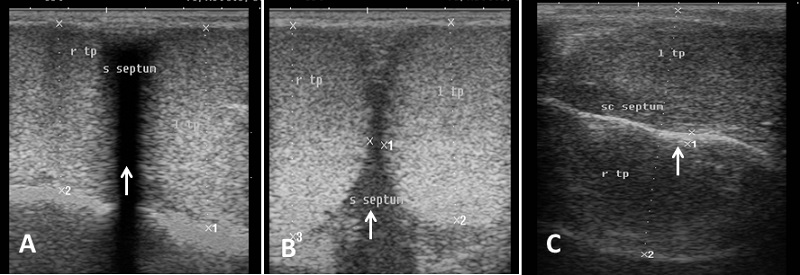
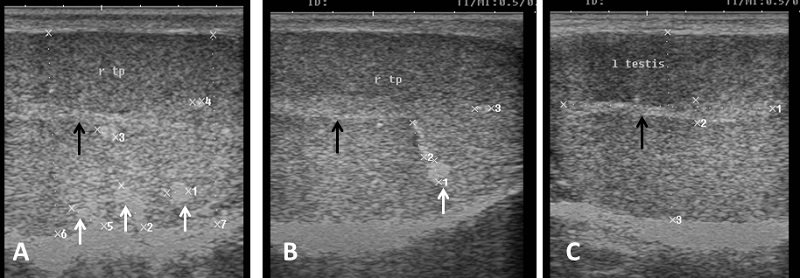
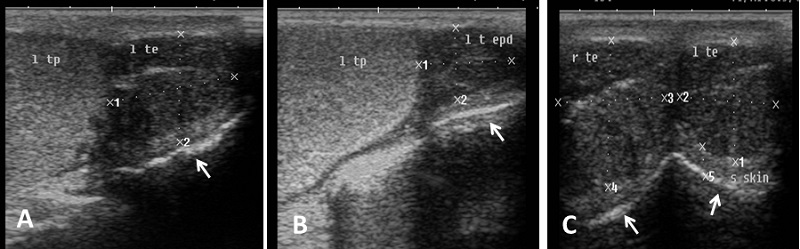
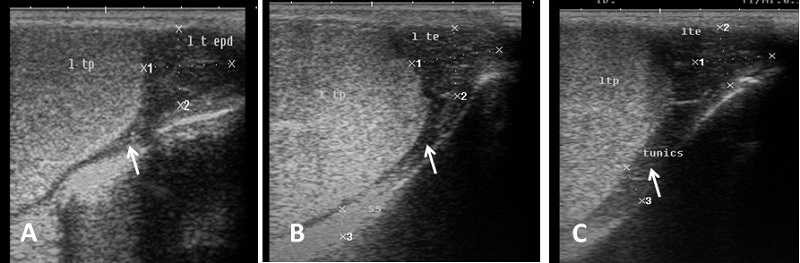
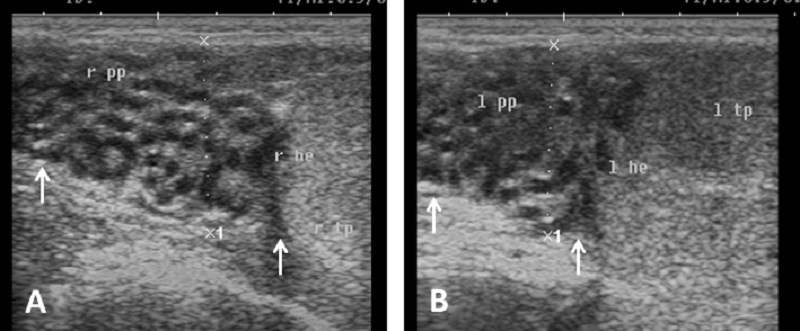
Echogenicity of the scrotum and its content Ultrasonographic characteristics of the testes revealed that the testicular parenchyma was homogeneous granular medium, which was gray to dark gray with a coarse medium echo-pattern. The borders of the testis were well circumscribed from the surrounding structures. The mediastinum testis was visualized as small or large white linear structure of greater echogenicity than testicular parenchyma and extended up to three quarters of the testicular length in five rams (5/8) and its echogenicity was varied between rams from moderate to diffuse (Fig. 1). The scrotal layer appeared very echogenic structure with presence of clear hypoechoic space or line separating it from the testis (Fig. 2). The cavum appeared between the parietal and visceral layer of tunica vaginalis as a non-echogenic line. The scrotal septum was highly echogenic line between the testes (Fig. 3). Scanning depth depended on the sizes of the testis and scrotum. During ultrasonographic examination only one ram (1/8) have uni-lateral testicular white hyper-echoic foci or spots scattered throughout the testicular parenchyma of the right testis, however, the left testis was normal in echogenicity (Fig. 4). The tail of the epididymis was clearly visible, less echoic than the testicular parenchyma and had a more heterogeneous structure with a hyperechoic line imaged in the center (Fig. 5). The head and body of the epididymis was imaged hardly in all rams. Furthermore, the body of the epididymis was imaged hypoechoic especially near the tail, which increased in its lumen (Fig. 6). The pampiniform plexus was clearly imaged as a dome-shaped structure, less echoic than the testicular parenchyma and containing numerous an-echoic black rounded or loop-like areas (Fig. 7).
Fig. 1. Ultrasound image of testes. (A) Homogeneous echo-pattern testicular parenchyma with large size central echogenic mediastinum testis. (B) Medium size central echogenic mediastinum testis. (C) Small size central echogenic and side radiating mediastinum testis (arrow).
Fig. 3. Simultaneous ultrasound image of both testes. (A) Dorsal section from caudal surface of the scrotum with presence of acoustic shadowing in the area of scrotal septum. (B) Cross section in the testes from caudal aspect show low hypo-echogenic septum (C) Dorsal section from lateral surface of the scrotum and the scrotal septum is imaged as hyperechoic line separating the testes (arrow).
Fig. 4. Ultrasound image of testes (A), (B) The parenchyma of right testis with slight hyper-echogenic white foci (white arrow) and mediastinum testis (black arrow). (C) The other left testis of the same ram appears normal in echogenicity and mediastinum (black arrow).
Fig. 5. Ultrasound image of tail of epididymis. (A) The tail of the epididymis clearly visible, less echoic than the testicular parenchyma with small white streaks (B) Tail of epididymis with hypoechogenic end part of epididymal body (C) Cross section in the right and left epididymal tail with ventral echogenic texture of the scrotal wall (arrow).
Fig. 6. Ultrasound image of body of epididymis. Note the hypoechogenicity of the body of epididymis near the tail than testicular parenchyma and increase its diameter (arrow).
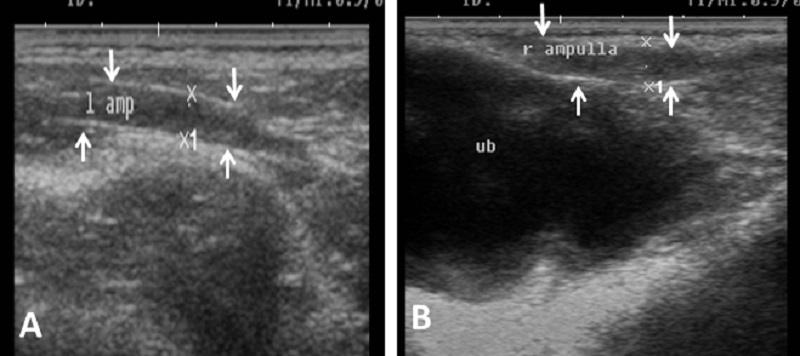
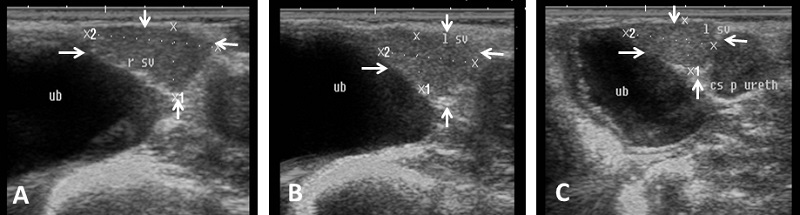
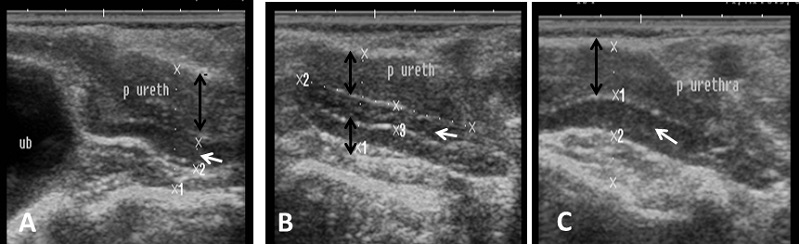
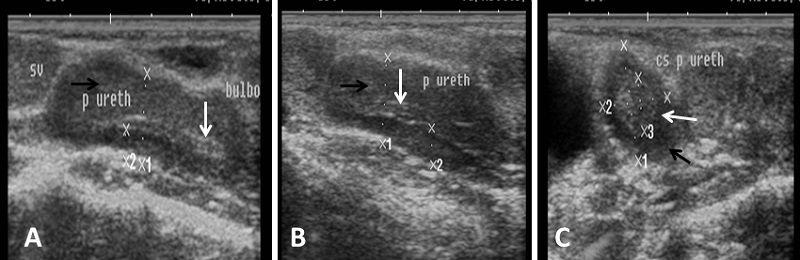
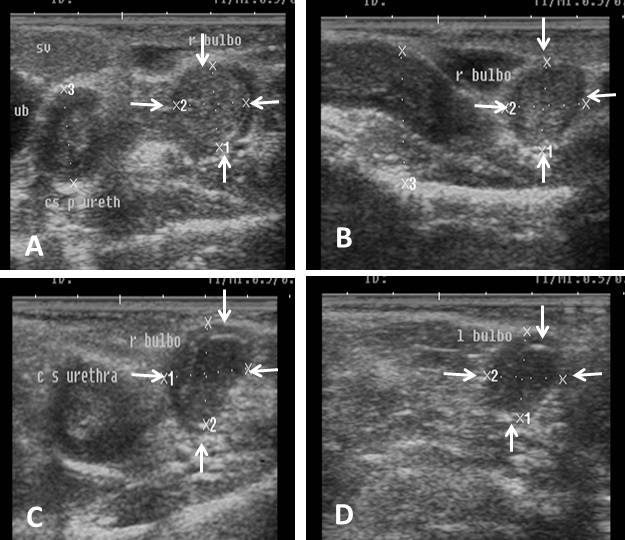
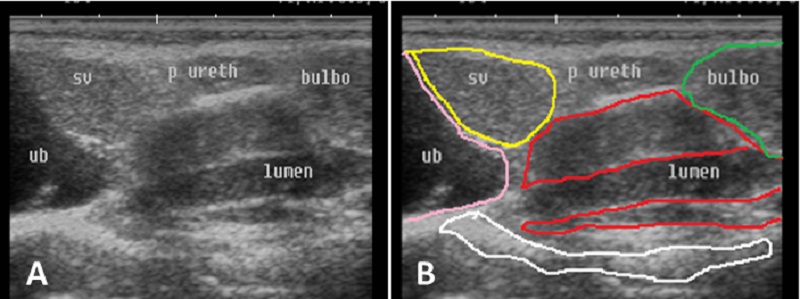
Fig. 7. Ultrasound image of pampiniform plexus. (A) Right pampiniform plexus (B) Left pampiniform plexus (arrow). Note the head of epididymis become hard to be visualized. Echogenicity of the accessory sex glands The ampulla appeared as two dorsal lumens on the neck of bladder with hypo-echoic to non-echogenic linear lumen. Glandular lining appeared as moderately echogenic line followed by hyper-echogenic muscular wall (Fig. 8). Vesicular glands were observed close to bladder (non-echoic) and appeared a heterogeneous hypo-echogenic structure with irregular outline and circumscribed with echogenic line (Fig. 9). The lumen of pelvic urethra appeared as non-echogenic tube, which was surrounded in the middle by moderately echogenic urethral muscle (Fig. 10). Pars disseminata of prostate was seen spread along the lumen of pelvic urethra from the urinary bladder to the region occupied by the bulbourethral glands and appeared slightly hypoechoic to moderate echogenic in relation to adjacent soft tissue with homogeneous echotexture (Fig. 11). Bulbourethral gland was oval in shape, its parenchyma appeared variable in echogenicity from hypo-echogenic to moderate echogenicity, and surrounded completely with low hypo-echogenic area and then with a white echogenic line that bounded it (Fig. 12). The pelvic urethra, urinary bladder, vesicular, pars disseminata, bulbourethral glands and bony pelvis were imaged in rams by rectal ultrasound probe (Fig. 13).
Fig. 8. Ultrasound image of ampulla. (A) Left ampulla (B) Right ampulla. Note the hypoechogenicity of lumen.
Fig. 9. Ultrasound image of vesicular glands. Note the hypoechogenicity of gland (arrow) and non-echogenicity black color of the urinary bladder.
Fig. 10. Ultrasound image of pelvic urethra. (A) Pelvic urethra show circumscribed lumen in the central which connect to the non-echogenic black urinary bladder (B) Pelvic urethra show urethral muscle enclosing lumen which have hypoechogenicity texture (black arrow). (C) Non-echogenic large lumen of pelvic urethra (white arrow) and more echogenic white color in the ventral part of image which indicates the bony floor of pelvis.
Fig. 11. Ultrasound image of pars disseminata of prostate. (A), (B) Longitudinal section in pelvic urethra show the echogenic part of prostate gland (pars disseminata) (white arrow) and hypoechogenicity of urethral muscle enclosing pelvic urethra (black arrow). (C) Cross section in pelvic urethra show urethral muscle (black arrow) surrounding pars disseminata of prostate (white arrow).
Fig. 12. Ultrasound image of bulbourethral glands. Note the variation in echogenicity of gland from low echogenic (A, B) to hypoechogenicity (C, D) and presence circular rim around gland with black non-echoic texture.
Fig. 13. Ultrasound image of accessory glands and pelvic urethra. Note the difference in echogenicity of urinary bladder, pelvic urethra, urethral muscle, pars disseminata of prostate gland, urethral lumen, seminal and bulbourethral gland. |
||||||||||||||||||||
|
|
||||||||||||||||||||
|
Discussion |
||||||||||||||||||||
|
Ultrasonographic examination of the testes, epididymis and accessory sex glands have been proved to be a valuable, non-invasive technique for the assessment of the morphology and pathology of the genitalia in several mammalian species including the ram (Gouletsou et al., 2003). Season could influence the testicular hemodynamics with changes in circulating concentrations of both testosterone and estradiol in Shiba goats (Samir et al., 2018). Data from the present study provided guidelines for ultrasonographic measurements of the testes and accessory sex glands in the non-breeding season in order to predict the future of reproductive performance of rams. In this study, the mean scrotum circumference was 28.87±1.1 cm and the morphometric measurements of the right and left testis length were 9.25±0.36 and 9.50±0.37 respectively. The testicular parenchyma was homogeneous granular with a coarse medium echo-pattern. Similar results were obtained by Cartee et al. (1990); Ahmad et al. (1991); Gouletsou et al. (2003); Sarker (2013) and Andrade et al. (2014). Also, Gouletsou (2017) reported that the echogenicity of the testes during the reproductive season were slightly reduced compared to that of non-breeding season. Furthermore, Ribeiro et al. (2017) concluded that the echogenicity of testicular parenchyma of pubertal animals increased in comparison with the pre-pubertal animals. The authors added that the testicular volume had significant correlation of high magnitude with scrotal circumference (0.91). In a recent study done by Hedia et al. (2019), there were no significant differences between the right and left testes with respect to testicular volume measurements and Doppler parameters of the testicular arteries. In the present study, the mediastinum testis appeared as a small or large white linear structure of greater echogenicity than the testicular parenchyma, which extended up to three quarters of the testicular length in five rams (5/8). In addition, its echogenicity varied between rams from moderate to diffuse in echogenicity. Similar results were obtained by Ahmad et al. (1991) and Gouletsou et al. (2003). Furthermore, Andrade et al. (2014) reported that its echogenicity and thickness have direct proportion with the animal age. Quantitative ultrasonographic parameters of testicular parenchyma were significantly correlated with its protein and lipid content in the ram (Ahmadi et al., 2013). In the present study, the scrotal layer appeared very echogenic structure with presence of clear hypoechoic space or line separating it from the testis and the scrotal septum visualized as a highly echogenic line between the testes. Similar results were obtained by Cartee et al. (1990); Ahmad et al. (1991); Gouletsou et al. (2003) and Andrade et al. (2014). The ultrasound assessment of the reproductive organs of males can help in diagnosis of diseases of the testicular parenchyma of adult rams, which can lead to subfertility. In the current study, only one ram (1/8) had a uni-lateral white hyper-echoic foci scattered throughout the testicular parenchyma of right testis, which may be attributed to slight degeneration with fibrosis. The testicular parenchyma appeared particularly echogenic, also there was an increase in the proportion of supportive connective tissue with acoustic shadowing. Similar results were obtained by Ahmad and Noakes (1995) and Gouletsou et al. (2006), Also, Sarker (2013) found some focal areas with white spots in one ram (1/5) that indicated chronic inflammation with calcification and it did not affect the semen quantity and quality. In the present study, the tail of the epididymis was clearly visible, less echoic than the testicular parenchyma and with a more heterogeneous structure. Furthermore, the body could be imaged especially near the tail with increased lumen. While, the head of the epididymis was imaged more heterogeneous and hypoechogenic than the nearby testicular parenchyma (Ahmad et al., 1991; Gouletsou et al., 2003). However, Sarker (2013) mentioned that the epididymis had the similar or slightly increased echogenicity as compared to the normal testis. In the current study, the pampiniform plexus was clearly imaged as a dome-shaped network structure, less echoic than the testicular parenchyma, containing numerous an-echoic rounded or loop-like areas, corresponding to spermatic vessels. Similar results were recorded by Ahmad et al. (1991) and Gouletsou et al. (2003). According to the authors knowledge, there are few published articles regarding the echogenicity and measurements of vesicular and bulbourethral glands in sheep (Ribeiro et al., 2017). Therefore, the findings of this study are important in assessing the reproductive performance of rams and can be used to aid in selection for future breeding. The ampullae appeared as two dorsal lines on the neck of the bladder with non-echogenic linear lumen. The glandular lining appeared as moderately echogenic line followed by hyperechogenic muscular wall. Vesicular glands were observed close to the urinary bladder (non-echoic) and appeared as a heterogeneous hypo-echogenic structure with irregular outline that circumscribed with echogenic line. Similar results were detected by Camela et al. (2017) and Ribeiro et al. (2017) and their topographic location and appearance fully matched those previously described in ruminant species (Setchell and Breed, 2006). Fully mature glands contain more plasma-storing micro-cystic structures dispersed within the parenchyma of the organ, which may result in the lower echogenicity of the seminal vesicles (Camela et al., 2017). The growth and differentiation of accessory sex glands are mainly regulated by androgens (Risbridger and Taylor, 2006). The obtained results revealed that pars disseminata of the prostate appeared slightly hypoechoic to moderate echogenic in relation to adjacent soft tissue with homogeneous echotexture. Similar results were obtained by Suri et al. (2009) and Camela et al. (2017). Bulbourethral gland was typical oval in shape and its parenchyma was varied in echogenicity from hypo-echogenic to moderate echogenicity. The gland was surrounded completely with low hypo-echogenic area and then with white echogenic line that bounded it. Results from this study are in accordance with those obtained by Camela et al. (2017) and Ribeiro et al. (2017) and also, with earlier findings of Webber et al. (1988) in rams and Santiago-Moreno et al. (2013) in the Iberian ibex. The dimension of the prostate gland was 12.9±1.2 compared with 14.2±2.7 mm and that of the bulbourethral gland was 13.7±1.3 compared with 14.7±1.8 mm, which were greater in sexually mature compared with peri-pubertal rams (Camela et al., 2017). The present study demonstrated the effect of early non-breeding season on the sonographic features of reproductive organs of Barki rams. |
||||||||||||||||||||
|
|
||||||||||||||||||||
|
Conclusion |
||||||||||||||||||||
|
The present study provides basic guidelines for the ultrasonography and measurements of the accessory sex glands and testes that can be used in the routine breeding soundness examination of Barki rams during early non-breeding season. The values of echogenicity and biometry of accessory sex glands can be used as complementary parameters to assist in the selection of rams with greater sexual activity. |
||||||||||||||||||||
|
|
||||||||||||||||||||
|
Acknowledgments |
||||||||||||||||||||
|
The researchers thank the staff of educational farm, Faculty of Veterinary Medicine, Sadat City University, Egypt, for providing all facilities to this study. |
||||||||||||||||||||
|
|
||||||||||||||||||||
|
Conflict of Interests |
||||||||||||||||||||
|
|
||||||||||||||||||||
|
The authors declared that no conflict of interests exists. |
||||||||||||||||||||
|
References |
||||||||||||||||||||
|
|
||||||||||||||||||||
|
Aboul-Naga, A.M. Aboul-Ela, M.B., 1987. Performance of subtropical Egyptian sheep breeds, European breeds and their crosses 1. Egyptian sheep breeds. World Review of Anim. Prod. 23, 75-82. Aboul-Naga, A.M., Aboul-Ela, M.B., Hassan, F., 1992. Manipulation of reproductive activity in subtropical sheep. Small Ruminant Research 7, 151-160. Ahmed, A.M., 2008. Biological evaluation of Barki sheep under two different breeding seasons. Egyptian J. Anim. Prod. 45, 15-24 Ahmed, N., Noakes, D.E., 1995. Seasonal variation in testes size, libido and plasma testosterone concentration in British goat. Anim. Sci. 61, 553–559. Ahmad, N., Noakes, D.E., Subandrio, A.L., 1991. B-mode, real time ultrasonographic imaging of the testis and the epididymis of sheep and goats. Vet. Rec. 128, 491-496. Ahmadi, B., Lau, C.P.S., Giffin, J., Santos, N., Hahnel, A., Raeside, J., Christie, H., Bartlewski, P., 2012. Suitability of epididymal and testicular ultrasonography and computerized image analysis for assessment of current and future semen quality in the ram. Exp. Biol. Med. 237, 186 -193. Ahmadi, B., Mirshahi, A., Giffin, J., Oliveira, M.E.F., Gaoa, L., Hahnel, A., Bartlewski, P.M., 2013. Preliminary assessment of the quantitative relationships between testicular tissue composition and ultrasonographic image attributes in the ram. The Veterinary Journal 198, 282–285. Andrade, A.K.G., Soares, A.T., Freitas, F.F., Silva, S.V., Pe˜na-Alfaro, C.E., Batista, A.M., Guerra, M.M.P., 2014. Testicular and epididymal ultrasonography in Santa Inés lambs raised in Brazil. Anim. Reprod. 11, 110–118. Camela, E.S.C., Nociti, R.P., Santos, V.J.C., Macente, B.I., Maciel, G.S., Feliciano, M.A.R., Vicente, W.R.R., Gill, I., Bartlewski, P.M., Oliveira, M.E.F., 2017. Ultrasonographic characteristics of accessory sex glands and spectral Doppler indices of the internal iliac arteries in peri- and post-pubertal Dorper rams raised in a subtropical climate. Anim. Reprod. Sci. 184, 29-35 Cartee, R.E., Rumph, P.F., Abuzaid, S., Carson, R., 1990. Ultrasonographic examination and measurement of ram testicles. Theriogenology 33, 867-875. Chandolia, R.K., Bartlewski, P.M., Omeke, B.C., Beard, A.P., Rawlings, N.C., Pierson, R.A., 1997. Ultrasonography of the developing reproductive tract in ram lambs: effects of a GnRH agonist. Theriogenology 48, 99–117. Gouletsou, P.G., 2017. Ultrasonographic examination of the scrotal contents in rams. Small Ruminant Research 152, 100–106. Gouletsou, P.G., Fthenakis, G.C., 2010. Clinical evaluation of reproductive ability of rams. Small Ruminant Research 92, 45-51 Gouletsou, P.G., Amiridis, G.S., Cripps, P.J., Lainas, T., Deligiannis, K., Saratsis, P., Fthenakis, G.C., 2003. Ultrasonographic appearance of clinically healthy testicles and epididymides of rams. Theriogenology 59, 1959-1972. Gouletsou, P.G., Fthenakis, G.C., Tzora, A., Cripps, P.J., Saratsis, P., 2006. Isolation of Arcanobacterium pyogenes from the scrotal skin and the prepuce of healthy rams or rams with testicular abnormalities. Small Ruminant Res. 63, 177–182. Hedia, M.G., El-Belely, M.S., Ismail, S.T., Abo El-Maaty, A.M., 2019. Monthly changes in testicular blood flow dynamics and their association with testicular volume, plasma steroid hormones profile and semen characteristics in rams. Theriogenology 123, 68-73. Hulet, C.V., Shupe, W.L., Ross, T., Richards, W., 1986. Effects of nutritional environment and ram effect on breeding season in range sheep. Theriogenology 25, 317-323. Islam, A.B.M., Land, R.B.C., 1977. Seasonal variations in testes diameter and sperm output of rams of breeds of different prolificacy. Anim. Prod. 25, 311–317. Kastelic, J.P., Cook, R.B., Pierson, R.A., Coulter, G.H., 2001. Relationships among scrotal and testicular characteristics, sperm production, and seminal quality in 129 beef bulls. Can. J. Vet. Res. 65, 111–115. Mahmoud, G.B., Abdel-Raheem, S.M., Hussein, H.A., 2013. Effect of combination of vitamin E and selenium injections on reproductive performance and blood parameters of Ossimi rams. Small Ruminant Research 113, 103– 108. Najjar, B., Benaoun, M., Ezzaouia, M., Mrad, B., 2012. Evaluation of Testicular Measurement and Sperm Production of Tunisian Arab Stallions using Ultrasonography. Asian J. Anim. Vet. Adv. 7, 205-209. Perez, C., Lopez, A., Castrillejo, A., Bielli, A., Laborde, D., Gastel, T., Tagle, R., Queirolo, D., Franco, J., Forsberg, M., Rodriguez-Martinez, H., 1997. Reproductive seasonality of Corriedale rams under extensive rearing conditions. Acta Vet. Scand. 38, 109-117. Ribeiro, M.S., Quirino, C.R., Junior, A.B., Pacheco, A., 2017. Biometry and ultrasound evaluation of testicles and accessory glands in Santa Ines rams. R. Bras. Zootec. 46, 317-323. Risbridger, G.P., Taylor, R.A., 2006. Physiology of the male accessory sex structures: the prostate gland, seminal vesicles, and bulbourethral glands. Knobil and Neill's Physiology of Reproduction, third edition. Elsevier. pp. 1149–1172. Samir, H., Nyametease, P., Nagaoka, K., Watanabe, G., 2018. Effect of seasonality on testicular blood flow as determined by color Doppler ultrasonography and hormonal profiles in Shiba goats. Animal Reproduction Science 197, 185–192. Santiago-Moreno, J., Toledano-Díaz, A., Castaño, C., Coloma, M.A., Esteso, M.C., Prieto, M.T., Delgadillo, J.A., López-Sebastián, A., 2013. Photoperiod and melatonin treatments for controlling sperm parameters, testicular and accessory sex glands size in male Iberian ibex: a model for captive mountain ruminants. Anim. Reprod. Sci. 139, 45–52. Sargison, N.D., 2008. Sheep Flock Health. A Planned Approach. Blackwell, Oxford. Sarker, S., 2013. Tesyiculr and epididymal ultrasonography for the assessment of semen quality in the indigenous ram. MS Thesis, Bangladesh Agricultural University. Schurich, M., Aigner, F., Frauscher, F., 2009. The role of ultrasound in assessment of male fertility. Eur. J. Obstet. Gynecol. Reprod. Biol. 144 Suppl 1, S192-198. Setchell, B.P., Breed, W.G., 2006. Anatomy, Vasculature, and innervation of the male reproductive tract. Knobil and Neill’s Physiology of Reproduction, third edition. Elsevier. pp. 771–825. Suri, S., Sudhakar, L.S., Bhardwaj, R.L., 2009. Anatomical studies of the prostate gland of Gaddi goat and sheep. J. Anim. Sci. 79, 294–296. Vanderperren, K., Stock, E., Pardon, B., Saunders, J., 2017. Contrast-enhanced ultrasound in sheep. Small Ruminant Research 152, 33–40. Webber, J.A., Hilt, C.J., Woods, G.L., 1988. Ultrasonographic appearance of bull accessory sex glands. Theriogenology 29, 1347–1355. |
||||||||||||||||||||
|
|