|
|
Journal of Advanced Veterinary Research Volume 9, Issue 1, 2019, Pages 14-22 |
|
|
Protective Effect of Avenanthramides against Cisplatin Induced Testicular Degeneration in Rats |
||||||||||||||||||||
|
|
||||||||||||||||||||
|
Yasmin O. El-Amir1,4, Doha Yahia2, Mohamed Samy Yousef 3 |
||||||||||||||||||||
|
|
||||||||||||||||||||
|
1Department of Pathology and Clinical Pathology, Faculty of Veterinary Medicine, Assiut University, Assiut, Egypt. 2Department of Forensic Medicine and Toxicology, Faculty of Veterinary Medicine, Assiut University, Egypt. 3Department of Theriogenology, Faculty of Veterinary Medicine, Assiut University, Assiut, Egypt. 4Department of Medical Laboratory Technology, College of Applied Medical Science, Jazan University, Jazan, KSA. |
||||||||||||||||||||
|
|
||||||||||||||||||||
|
Received 1 November 2018, Accepted 3 January 2019 |
||||||||||||||||||||
|
|
||||||||||||||||||||
|
Abstract |
||||||||||||||||||||
|
This study was performed to evaluate the protective effect of Avenanthramides (AVA) against testicular degeneration induced by cisplatin (CP) in rats. Thirty six male rats were equally divided into four groups: Group I served as control group, group II received intraperitoneal injection of CP at a dose of 7 mg/kg b.wt., group III received AVA (20 mg/kg b.wt.) daily for one week before injection of CP, group IV received only AVA. Administration of CP reduced sperm count, motility and viability, testicular weight and epithelial height. CP administration increased sperm DNA damage and malondialdehyde (MDA) level. The level of glutathione peroxidase and testosterone hormone were decreased in CP group. Severe histopathological changes in testes of CP exposed rats were prominent. On the other hand, administration of AVA could improve the sperm viability, sperm count and testicular weight and reduced the degree of DNA damage in sperms. AVA reduced the level of MDA and the histopathological changes in testes. In conclusion, AVA can reduce the oxidative stress and ameliorate the degenerative changes in testes induced by CP. |
||||||||||||||||||||
|
|
||||||||||||||||||||
|
Keywords: |
||||||||||||||||||||
|
|
||||||||||||||||||||
|
Avenanthramides, Cisplatin, Oxidative stress, Testicular damage |
||||||||||||||||||||
|
Introduction |
||||||||||||||||||||
|
Cisplatin (CP) is a chemotherapeutic agent used for treatment of wide variety of cancers including testicular tumors (Duale et al., 2007), squamous cell carcinoma of head and neck (Baur et al., 2002), small cell lung cancer (Noda et al., 2002), ovarian cancer (Lambert and Berry, 1985) and invasive bladder cancer (Shipley et al., 1987). It has been shown that CP induced hepatotoxicity (Waseem et al., 2015), nephrotoxicity (dos Santos et al., 2012; Purena et al., 2018) and testicular degeneration (Zhang et al., 2001). It reduced the number of epithelial cells in seminiferous tubules, count of sperms and sperm viability after a single intraperitoneal injection at the dose of 7 mg/kg. b.wt in rats (Ateşşahin et al., 2006; Sherif et al., 2014). Furthermore, Apoptosis of germ cells was induced in mice after a single intraperitoneal injection of CP of either 1, 5, or 10 mg/kg (Zhang et al., 2001). Reactive oxygen species were implicated in the pathogenesis of CP induced testicular degeneration (Ilbey et al., 2009). CP increased lipid peroxidation and decreased the activity of antioxidant enzymes (Türk et al., 2008). Oat (Avena sativa), a cereal grain, that is concerned for its health benefits (Xu et al., 2013) and antioxidants activity (Chen et al., 2007). Oats contain many phytochemicals such as tocotrienols, phenolic acids, flavonoids, sterols and phytic acid (Peterson, 2001). Avenanthramide (AVA) is a nitrogen containing phenolic compound described by Collins (1989). AVA is exclusive to oats and not found in other cereal grains. AVA represents a chief part of the total phenolic compound in oats (Chu et al., 2013). There are many forms of AVA. The most common forms are AV2c, AV2f and AV2p, which are known for their strong antioxidant activity (Emmons et al., 1999). AVA increases the activity of antioxidant enzymes in liver and kidney of rat (Ji et al., 2003). AVA has also an anti-inflammatory activity. It was suggested that AVA decreases the production of cytokines through inhibition of nuclear factor kappa B activation (Guo et al., 2008). Oat oil can reduce lipid peroxidase and enhanced density, motility and morphology of sperms in mice induced by deltamethrin (Halima et al., 2014). This study aimed to investigate the protective effect of AVA against testicular injury induced by CP in Sprague-Dawely rats. |
||||||||||||||||||||
|
|
||||||||||||||||||||
|
|
||||||||||||||||||||
|
Materials and methods |
||||||||||||||||||||
|
Animals Thirty six male adult Sprague Dawley rats (weighing 150-200 gm) were purchased from animal house, Faculty of Medicine, Assiut University, Egypt. The animals were left for one week for acclimatization. Animals were kept under standard conditions (12 hrs dark/light cycle, 22.0±3.0°C and 60% humidity). Food and water were available ad libitum. This experiment was conducted according to the guidelines and ethics of animal handling and care at Assiut University, Egypt. Chemicals Cisplatin (1mg/ml) obtained from Mylan S.A.S., Priest, France and AVA enriched extract was obtained from Irvene, California, USA (C25H31F3O5S). Experimental design Rats were divided randomly and equally into 4 groups (n=9 per group). Group I served as control group. Group II (CP group) received a single intraperitoneal injection of CP at a dose of 7mg/kg b.wt. (Ilbey et al., 2009). Group III (AVA+CP group) received AVA at a dose of 20 mg/kg b.wt. by gastric tube daily for one week before single intraperitoneal injection of CP (7 mg/kg b.wt.). Group IV (AVA group) received only AVA at a dose of 20 mg/kg b.wt. by gastric tube daily for one week. Blood samples were collected from three rats from each group at 24hrs, 48hrs, and 72hrs following injection of CP in groups II and III, and after one week in group IV. Weight of the body was measured, then the two testes were dissected, and weighed. Relative weight of the testes was evaluated using the following formula: Relative weight of testes= Weight of testes (g)/weight of the body (g) x100 Blood samples Blood samples were collected by retro-orbital bleeding into plain tubes, serum were obtained according to Coles (1986), and used for measurement of malondialdehyde level (MDA), glutathione peroxidase activity (GPX) and testosterone level. Collection of sperms The cauda epididymis was excised, minced and incubated in a pre-warmed petri dish containing 10 mL Hank’s balanced salt solution at 37°C. After few minutes, the cauda epididymis was discarded, and the recovered suspension was gently shaken and then used for sperm analysis (Rashidi et al., 2004; Mesbah et al., 2007). Sperms from the cauda epididymis were used immediately for sperm analysis and acridine orange assay. Evaluation of sperms Sperm count For sperm count, 500 μL of the recovered suspension was diluted with formaldehyde fixative solution (10% formalin in PBS). Approximately 10 μL from the diluted solution was transferred into a haemocytometer (Thoma, assistant Sondheim/Rhön, Germany) and let to stand for few minutes. Then the settled sperms were counted per 250 small squares of a haemocytometer (Seed et al., 1996). Sperm Viability Viability was assessed by eosin Y staining (5% in saline). Forty microliter samples of the fresh sperm suspension were placed on a glass slide, mixed with 10 μL eosin and observed under a light microscope (400x magnification). Live sperms remained colorless; while, dead sperm stained by the pink color. At least 200 sperms were counted from each sample in ten fields of vision randomly, and the percentage of live sperms was recorded (Björndahl et al., 2003). Sperm Motility Sperm motility was assessed by visual estimation (100 sperm, in duplicate) under a phase-contrast microscope at 200x magnification. Sperms were classified as: stable, motile without progression and motile with progressive movement (Seed et al., 1996). Sperm DNA integrity using acridine orange staining assay Acridine orange test (AOT) is a simple microscopic method based on acid conditions to denaturant DNA followed by staining with acridine orange. AO intercalates into native DNA and the dye fluoresces green when exposed to blue light and fluoresces yellow to red when it binds to the fragmented DNA (Varghese et al., 2011). The assay was performed according to Tejada et al. (1984) with some modifications; thick smears of sperm were air dried then soaked in Carnoy’s fixative (methanol: acetic acid 3:1) for 2 hrs. Slides were rinsed with water then stained with AO stain [1 ml of 1% AO in distilled water + 90 ml of 10% acetic acid + 10 ml of Na2Hpo4 (2.83 g in 100 ml distilled water)] for 5 minutes. Then, the slides were rinsed with deionized water and left to dry at room temperature. Slides were examined under fluorescent microscope (Olympus BX 43, Japan) equipped with blue filter. Two- hundred sperms were analyzed per slide. Biochemical assays Determination of serum malondialdehyde level (MDA) Determination of serum MDA was done according to Grotto et al. (2009) by using colorimetric kit supplied by Bio-diagnostics (Dokki, Giza, Egypt). The assay was performed according to the instructions of the kit, absorbance was measured at 534 nm by spectrophotometric means (UV spectrophotometer, Optizen 3220 UV, Mecasys Co. Ltd, Korea). Determination of serum glutathione peroxidase activity (GPX) Activity of glutathione peroxidase in serum samples was measured according to the method of Paglia and Valentine (1967) by using test kits supplied by Bio-diagnostic (Dokki, Giza, Egypt), the analysis was done according to the kit protocol, absorbance was measured by UV spectrophotometer (Optizen 3220 UV, Mecasys Co. Ltd, Korea) at 340 nm. Determination of serum testosterone level Hormonal analysis of serum testosterone level was determined by enzyme-linked immunosorbent assay (ELISA) using testosterone commercial kits (DRG diagnostics, GmbH, Germany). A microtiter plate reader (Stat Fax - 2100, Awareness, Technology Inc., USA) provided with printer EPSON-LX 300+ was used to read the ELISA plate. Histopathological examination Specimens from both testes were fixed in neutral buffered formalin, dehydrated by alcohol, cleared by xylene and embedded in paraffin blocks. The paraffin blocks were cut at 5 µm and stained with hematoxylin and eosin (H&E) according to Bancroft et al. (1996). Round seminiferous tubules were elected. The height of the germinal epithelium was measured in ten randomly selected fields of each testicle using research microscope Axiostar plus type made by Zeiss which connected to professional digital image analysis system. Statistical analysis Data are expressed as the mean ± SE for all parameters. The data were analyzed using the SPSS software package version 16.0. For difference between groups, one-way analysis of variance (ANOVA) test was performed followed by Post Hoc Lowest Significant Difference (LSD) multiple range test. P<0.05 and P<0.01 was considered significant compared to control. |
||||||||||||||||||||
|
|
||||||||||||||||||||
|
Results |
||||||||||||||||||||
|
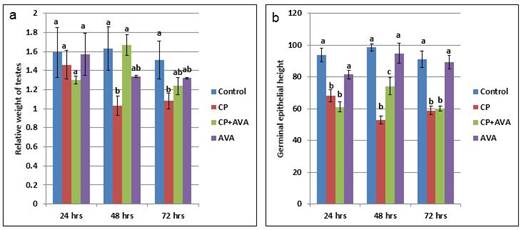
Effect on testicular weight The relative testicular weight was significantly decreased (P< 0.01) at 48 and 72 hrs in CP group in comparison with control group. Co-administration of AVA improved the relative testicular weight at 48 hrs (Fig. 1a).
Fig. 1. Relative weight of testes and height of germinal epithelium in control, CP, AVA+CP and AVA groups at 24 hrs, 48 hrs, and 72 hrs. (a) Relative weight of testes. (b) Height of germinal epithelium. Different letters a, b and c indicate significance among groups. Effect on sperm count The sperm count was significantly decreased (P< 0.01) in CP exposed groups in a time dependent manner when compared with the control group. Administration of AVA could not modify the effect of CP at 24hrs but significantly (P< 0.01) increased the sperm count at 48 and 72 hrs time points (Table 1). Effect on sperm motility Cisplatin reduced the sperm motility. AVA treatment could increase the sperm motility at 24, 48 and 72 hrs (Table 1). Table 1. Sperm parameters in control, CP, AVA+CP and AVA groups at 24 hrs, 48 hrs, and 72 hrs.
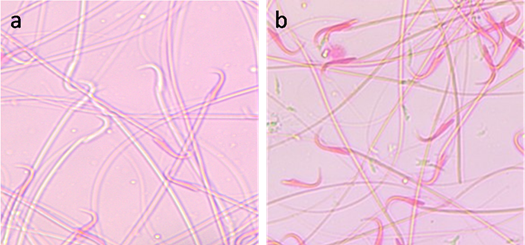
Data are presented as Mean±SE In each column values followed by different superscript is significant (P<0.05) CP: Cisplatin; AVA: Avenanthramides; AO: Acridine orange test Effect on sperm viability Exposure to CP for 24, 48 and 72 hrs significantly (P<0.01) reduced the sperm viability in a time related manner. Administration of AVA significantly (P<0.05) increased the sperm viability percentage at 24, 48 and 72 hrs compared with CP group (Table 1 and Fig. 2).
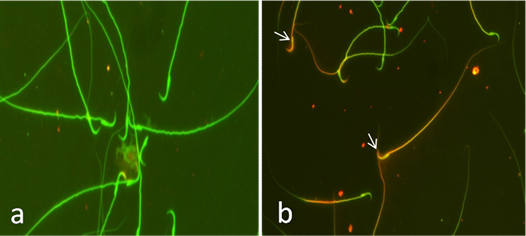
Fig. 2. Sperm viability assessed by eosin Y staining. (a) Viable sperm. (unstained head). (b) Dead sperms (stained head). Eosin Y stain (x100). Acridine orange DNA damage assay Exposure of rats to CP induced a significant increase in sperm DNA damage at 24, 48 and 72 hrs (P< 0.05) indicated by increased the number of sperms with yellow and orange head compared with the number of sperms with undamaged DNA that appeared with green head. Co-administration of AVA with CP reduced the degree of DNA damage in sperms in time dependent manner (P< 0.05) while rats exposed to AVA only showed the same degree as control ones (Table 1 and Fig. 3).
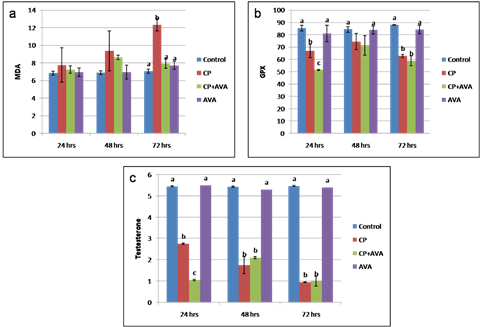
Fig. 3. Sperm integrity using acridine orange test (AOT). (a) Sperms with undamaged DNA showing green head. (b) Sperms with damaged DNA indicated by yellow or orange head (arrows). Findings of the biochemical analyses Serum malondialdehyde (MDA) level Cisplatin at 72 hrs significantly increased the lipid peroxidation expressed by increased MDA level (P< 0.01). Administration of AVA significantly reduced the level of MDA at 24, 48 and 72 hrs (P< 0.001) (Fig. 4a). Glutathione peroxidase level Glutathione peroxidase level was decreased at 24 hrs (P< 0.05) and 72 hrs (P<0.01) in CP group. AVA administration was not effective (Fig. 4b). Serum testosterone hormone level Testosterone hormone was decreased (P< 0.01) in all exposed groups. Co-administration of AVA could not improve the level of testosterone (Fig. 4c).
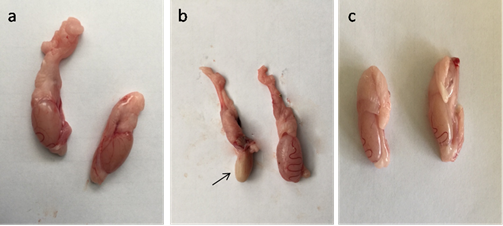
Fig. 4. Serum level of MDA, GPX and testosterone in control, CP, AVA+CP and AVA groups at 24 hrs, 48 hrs, and 72 hrs. (a) MDA level. (b) GPX level. (c) Testosterone level. Different letters a, b and c indicate significance among groups. Gross pathological findings Examination of control testes revealed normal testicular size with no gross lesion (Fig. 5a). Administration of CP significantly decreased size of testes. Complete atrophy of testes was observed in one rat 72 hrs following injection of CP (Fig. 5b). Co-administration of AVA resumed the weight of testes (Fig. 5c).
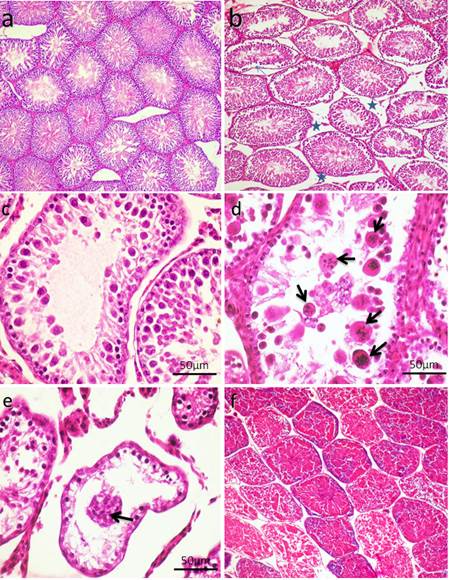
Fig. 5. Photomicrograph of testes. (a) Control group. (b) CP group. Note, complete atrophy of the right testes (arrow). (c) AVA+CP group. Histopathological findings The testicular epithelial height was decreased at 24, 48 and 72 hrs (P<0.01) when compared with control group. AVA administration improved the effect of CP only at 48 hrs (Fig. 1b). Examination of control testes revealed normal tissue architecture (Fig. 6a). Interstitial edema and mild testicular degeneration were observed 24 hrs after injection of CP. The testicular changes were represented by decrease in number of spermatogenic cells in some seminiferous tubules without empty lumen (Fig. 6b). After 48 hrs there was severe exhaustion of the spermatogenic epithelium. The lumen of the seminiferous tubules was empty and most of tubules were lined with only spermatogonia and primary spermatocytes (Fig. 6c). Spermatid giant cells were observed in some seminiferous tubules. The cells were characterized by eosinophilic cytoplasm and multiple pyknotic nuclei (Fig. 6d). After 72 hrs, most of the tubules were completely empty and lined only by sertoli cells (Fig. 6e). In one case, there was a complete necrosis of the seminiferous tubules. The tubules were filled with granular eosinophilic debris in association with mineralization (Fig. 6f).
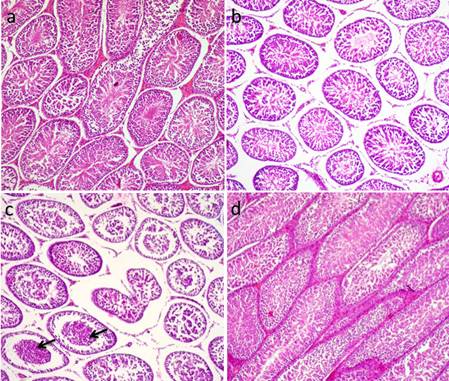
Fig. 6. Photomicrograph showing control testes and histopathological changes of testes at 24 hrs, 48 hrs and 72 hrs following injection of CP. (a) Control group. Normal testicular architecture. (b) CP group, 24 hrs following injection. Interstitial edema (stars). (c-d) CP group 48hrs following injection. (c) Exhaustion of spematocytes. Only few layers of spermatogenic epithelium could be seen, spermatogonia and primary spermatocytes. (d) Formation of spermatid giant cells (arrows). (e-f) CP group 72 hrs following injection of CP. (e) Empty seminiferous tubules lined only with spermatogonia. (f) Dead tissue in the lumen of the seminifrous tubules and mineralization (H&E). Administration of AVA one week before CP injection countercurrent the histopathological changes induced by CP to some level. Most of the seminiferous tubules were normal 24 hrs post injection of CP (Fig. 7a). Interstitial edema and mild degenerative changes were observed 48 hrs (Fig. 7b). After 72hrs, the height of the germinal epithelium was reduced with the presence of desquamated epithelium in the lumen of some tubules (Fig. 7c). No changes can be observed in AVA group (Fig. 7d).
Fig. 7. Photomicrograph showing histopathological picture of AVA+CP group and AVA group. (a) AVA+CP group after 24 hrs. Normal seminiferous tubules. (b) AVA+CP group after 48 hrs. Interstitial edema and mild degenerative changes. (c) AVA+CP group after 72 hrs. Necrosed tissue in the lumen of some tubules (arrow). (d) AVA group. Normal testes. (H&E). |
||||||||||||||||||||
|
|
||||||||||||||||||||
|
Discussion |
||||||||||||||||||||
|
Despite of the toxic effect of CP on kidney (Purena et al., 2018), liver (Omar et al., 2016) and testes (Reddy et al., 2016), CP is one of the most common chemotherapeutic agents against many types of neoplasms. In the current study, intraperitoneal injection of CP at a dose of 7 mg/kg. b.wt. adversely affected different sperm parameters when compared with control group. These results are constant with previous studies which found that injection of CP reduced count and motility of sperms (Ateşşahin et al., 2006; Reddy et al., 2016). It was shown that CP reduced testicular weight and height of the germinal epithelium. These results were recently observed by Singh et al. (2017b). It was reported that injection of CP at dose of 10 mg/kg in mice induced germ cell loss and increased the number of dead germ cells, which decreased the size of the testes (Cherry et al., 2004). The reduction in sperm count, testicular weight and height of the germinal epithelium may be related to the severe degenerative changes of testes, which can be demonstrated histopathologically. In the present study, the degenerative changes caused by CP exposure were in the form of severe exhaustion of germinal epithelium, the presence of spermatid giant cells, which extended to complete necrosis of some seminiferous tubules. These results are compatible with previous investigators, who experienced severe degenerative changes of testes following injection of CP (Mohammadnejad, 2012; Liu et al., 2015). It was reported that chemotherapeutic agents lead to damage of the epithelial lining seminiferous tubules and subsequently resulted in oligospermia or azoosperemia (Ateşşahin et al., 2006). Other investigators reported the decrease in mitosis following treatment with chemotherapeutic drugs. (Khilkevich and Kurilo, 1992). In the same way, it was found that chemotherapy acts on rapidly dividing cells and stop their division (Howell and Shatel, 2001). The toxicity of CP can be also attributed to the steroidogenesis suppression and the release of reactive oxygen species (ROS) (Ahmed et al., 2011). Testosterone hormone was significantly decreased in CP group (P< 0.01). This reduction was in agreement with Ilbey et al. (2009). It was reported that the reduction of testosterone level resulting from the harmful effect of CP on leydig cells. Aydiner et al. (1997) explained that the reduction of testosterone level after administration of 5 mg/kg.b.wt. of CP may be attributed to the damage of Leydig cells and the reduction in the effects of the germinal epithelium on the LH-Leydig cell axis. Another hypothesis was reported that CP inhibits testosterone synthesis by the inhibition of cholesterol side-chain cleavage enzyme (P450scc), which is responsible for the process of steroidogenesis (García et al., 2012). Injection of CP showed a significant increase in sperm DNA damage at 24, 48 and 72 hrs (P< 0.05). This result is in agreement with results of Singh et al. (2017a) who found that injection of CP at a dose of 7.5 mg/kg. b.wt. for four weeks induced DNA fragmentation, which can be detected by ethidium bromide/acridine orange staining. It was found that the mode of action of CP is the formation of DNA adducts, which results in DNA fragmentation (Yousef and Hussien, 2015). The accumulation of DNA adducts following administration of CP in a dose dependant manner was demonstrated by Hooser et al. (2000). DNA adducts induced by CP may be accumulated in the testes (Poirier et al., 1992). In this study, cisplatin significantly increased MDA level at 72 hrs (P<0.01). Many authors have reported the increase of MDA in testes following administration of CP (Said Salem et al., 2017; Singh et al., 2017a). The increase of MDA may be related to DNA fragmentation, which has been reported in the present study. CP also decreased GPX level at 24 hrs (P< 0.05) and 72 hrs. These results are in agreement with previous studies (Ateşşahin et al., 2006; Salem et al., 2012; Reddy et al., 2016). It has been reported that the decrease of GPX activity increase the susceptibility of sperms to free radicals (Vernet et al., 2004), as GPX antioxidant acts as a scavenger of free radicals (Abd Ellah et al., 2004). In the current study, administration of AVA at dose of 20 mg/kg b.wt, one week before injection of CP increased the sperm count and sperm motility at 48 and 72 hrs and increased the sperm viability percentage at all times compared with CP group. AVA also improved the effect of CP on testicular weight and height of testicular epithelium. AVA also reduced and delayed the histopathological changes induced by CP. It was found that administration of oats oil could improve the biochemical parameters and the histopathological changes induced by deltamethrin in testis of mice (Halima et al., 2014). These results may be related to the reduction of MDA by administration of AVA, which had been reported in the present study. Liu et al. (2011) reported the decrease of plasma MDA after A. Sativa extract administration for 1 month. It has been found that A. Sativa extracts acquired antioxidant effect in vitro by 2,2-di(4-tert-octylphenyl)-1-picrylhydrazyl and beta carotene bleach assays (Bratt et al., 2003; Fagerlund et al., 2009). Oat oil decreased the detrimental effect of oxygen species, which causes sperm membrane damage (Baumber et al., 2000). It was found that the antioxidant ability of AVA is 10–30 times more than that of phenolic compounds of oats (Bratt et al., 2003). Hassanein and El-Amir (2017) found that AVA significantly decreased DNA damage through its strong antioxidant effect of AVA. The level of GPX was low in AVA+ CP group. These results were in agreement with Kaviarasan et al. (2008), who stated that the low levels of non-enzymatic antioxidants may be attributable to increased their consumption to scavenge free radicals. |
||||||||||||||||||||
|
|
||||||||||||||||||||
|
Conclusions The present study clearly reported the degenerative changes in testes induced by cisplatin. Co-administration of AVA with CP ameliorates the histopathological changes and testicular function by reduction of oxidative stress. These results can suggest the consideration of AVA as protective agent for the male fertility during CP administration. |
||||||||||||||||||||
|
|
||||||||||||||||||||
|
Conflict of Interests |
||||||||||||||||||||
|
|
||||||||||||||||||||
|
The authors have declared that no competing interests exist. |
||||||||||||||||||||
|
References |
||||||||||||||||||||
|
Abd Ellah, M.R., Nishimori, K., Goryo, M., Okada, K., Yasuda, J., 2004. Glutathione peroxidase and glucose 6 phosphate dehydrogenase activities in bovine blood and liver. J. Vet. Med. Sci. 66,1219-1221. Ahmed, E.A., Omar, H.M., Ragb, S.M., Nasser, A.Y., 2011. The antioxidant activity of vitamin C, DPPD and L-cysteine against cisplatin-induced testicular oxidative damage in rats. Food Chem. Toxicol. 49,1115-1121. Ateşşahin, A., Karahan, I., Türk, G., Gür, S., Yılmaz, S., Çeribas, A.O., 2006. Protective role of lycopene on cisplatin-induced changes in sperm characteristics, testicular damage and oxidative stress in rats. Reprod. Toxicol. 21, 42-47. Aydiner, A., Aytekin, Y., Topuz, E., 1997. Effects of cisplatin on testicular tissue and the Leydig cell-pituitary axis. Oncol. 54, 74-78. Bancroft, T.D., Stevens, A., Turner, D.R.,1996. Theory and Practice of Histological Technique, 4th ed., Churchill Livingstone, New York, USA, pp. 113-139. Baumber J., Ball B.A., Gravence, C.G., Medina, V., Davies Morel, M.C., 2000. The effect of reactive oxygen species on equine sperm motility, viability, acrosomal integrity, mitochondrial membrane potential, and membrane lipid peroxidation. J. Androl. 21, 895-902. Baur, M., Kienzer, H.R., Schweiger, J., DeSantis, M., Gerber, E., Pont, J., Hudec, M., Schratter-Sehn, AU, Wicke, W, Dittrich, C., 2002. Docetaxel/cisplatin as first-line chemotherapy in patients with head and neck carcinoma: a phase II trial. Cancer 94, 2953-2958. Björndahl, L., Söderlund I., Kvist U., 2003. Evaluation of the one step eosin‐nigrosin staining technique for human sperm vitality assessment. Hum. Reprod. 18, 813-816. Bratt, K., Sunnerheim, K., Bryngelsson, S., Fagerlund, A., Engman, L., Andersson, R.E., 2003. Avenanthramides in oats (Avena sativa L.) and structure– antioxidant activity relationships. J. Agric. Food Chem. 51, 594-600. Chen, C.Y.O., Milbury, P.E., Collins, F.W., Blumberg, J.B. 2007. Avenanthramides are bioavailable and have antioxidant activity in humans after acute consumption of an enriched mixture from oats. J. Nutr. 137, 1375-1382. Cherry, S.M.., Hunt, P.A., Hassold, T.J., 2004. Cisplatin disrupts mammalian spermatogenesis, but does not affect recombination or chromosome segregation. Mutat. Res. 564, 115-128. Chu, Y.F., Wise, M.L., Gulvady, A.A., Chang, T., Kendra, D.F., Jan-Willem van Klinken, B., Shi, Y., O'Shea, M., 2013. In vitro antioxidant capacity and anti-inflammatory activity of seven common oats. Food chem. 139, 426-431. Coles, E.H., 1986. Veterinary Clinical Pathology. 4th Ed. W.B. Saunders Co., Philadelphia, London, p.183. Collins F.W. 1989. Oat phenolics: Avenanthramides, novel substituted N-cinnamoylanthranilate alkaloids from oat groats and hulls. J. Agric. Food Chem. 37, 60-66. dos Santos, N.A.G., Rodrigues, M.A.C., Martins, N.M., dos Santos, A.C., 2012. Cisplatin-induced nephrotoxicity and targets of nephroprotection: an update, Arch. Toxicol. 86, 1233-1250. Duale, N., Lindeman, B., Komada, M., Olsen, A-K., Andreassen, A., Soderlund, E.J., Brunborg, G., 2007. Molecular portrait of cisplatin induced response in human testis cancer cell lines based on gene expression profiles. Mol. Cancer 6, 53. Emmons, C.L., Peterson, D.M., Paul, G.L. 1999. Antioxidant capacity of oat (Avena sativa L.) extracts. 2. In vitro antioxidant activity and contents of phenolic and tocol antioxidants. J. Agric. Food Chem. 47, 4894-4898. Fagerlund, A., Sunnerheim, K., Dimberg, L.H., 2009. Radical-scavenging and antioxidant activity of avenanthramides. Food Chem. 113, 550-556. García, M.M., Acquier, A., Suarez, G, Gomez, N.V., Gorostizaga, A., Mendez, C.F., Paz, C., 2012. Cisplatin inhibits testosterone synthesis by a mechanism that includes the action of reactive oxygen species (ROS) at the level of P450scc. Chem. Biol. Interact. 199, 185-191. Grotto, D., Maria, L.S., Valentini, J., Paniz, C., Schmitt, G., Garcia, S.C., Pomblum, V.J., Rocha, J.B.T., Farina, M., 2009. Importance of the lipid peroxidation biomarkers and methodological aspects FOR malondialdehyde quantification. Quím Nova 32, 169-174. Guo, W., Wise, M.L., Collins, F.W., Meydani, M., 2008. Avenanthramides, polyphenols from oats, inhibit IL-1β-induced NF-κB activation in endothelial cells. Free Radic. Biol. Med. 44, 415-429. Halima, N.B., Slima, A.B., Moalla, I, Fetoui, H., Pichon, C., Gdoura, R., Abdelkafi S., 2014. Protective effects of oat oil on deltamethrin induced reprotoxicity in male mice. Food Funct. 5, 2070-2077. Hassanein, K.M.A., El-Amir, Y.O., 2017. Protective effects of thymoquinone and avenanthramides on titanium dioxide nanoparticles induced toxicity in Sprague-Dawley rats. Pathol. Res. Pract. 213, 13-22. Hooser, S.B., van Dijk-Knijnenburg, W.C., Waalkens-Berendsen, I.D., Smits-van Prooije, A.E., Snoeij, N.J., Baan, R.A., Fichtinger-Schepman, A.M., 2000. Cisplatin-DNA adduct formation in rat spermatozoa and its effect on fetal development. Cancer Lett. 151, 71-80. Howell S.J., Shatel S.M., 2001. Testicular function following chemotherapy. Hum. Reprod. Update 7, 363-369. Ilbey, Y.O., Ozbek, E., Cekmen, M., Simsek, A., Otunctemur, A., Somay, A., 2009. Protective effect of curcumin in cisplatin-induced oxidative injury in rat testis: mitogen-activated protein kinase and nuclear factor-kappa B signaling pathways. Hum. Reprod. 24, 1717-1725. Ji, L.L., Lay, D., Chung, E., Peterson, D., 2003. Effect of avenathramides on oxidant generation and antioxidant enzyme activity in exercised rats. Nutr. Res. 23, 1579-1590. Kaviarasan, S., Sundarapandiyan, R., Anuradha C.V., 2008. Protective action of fenugreek (Trigonella foenum graecum) seed polyphenols against alcohol-induced protein and lipid damage in rat liver. Cell Biol. Toxicol. 24, 391-400. Khilkevich, L.V., Kurilo, L.F. 1992. The gametatoxic effect of antenatal exposure to thiotepa in mice. Ontogenez 23, 401-406. Lambert, H.E., Berry, R.J., 1985. High dose cisplatin compared with high dose cyclophosphamide in the management of advanced epithelial ovarian cancer (FIGO stages III and IV): report from the North Thames Cooperative Group. Br. Med. J. 290, 889-893. Liu, S., Yang, N., Hou, Z-H., Yao, Y., Lü, L., Zhou, X-R., 2011. Antioxidant effects of oats avenanthramides on human serum. Agric. Sci. China 10, 1301-1305. Liu, Z., Sun, Y., Su, L., Sun, Y., Kong, S., Chang, X., Guo, F., Li, W., Guo, J., Li, J., 2015. Effects of cisplatin on testicular enzymes and Sertoli cell function in rats. Fund Toxicol. Sci. 2, 137-145. Mesbah, S., Shokri, S., Karbalay-Doust, S., Mirkhani, H., 2007. The effect of nandrolone decanoate on the body, testis and epididymis weight and semen parameters in adult male rats. Iran J. Med. Sci, 32, 93-99. Mohammadnejad, D., Abedelahi, A., Soleimani-rad, J., Mohammadi-roshandeh, A., Rashtbar, M., Azami, A., 2012. Degenerative Effect of Cisplatin on Testicular Germinal Epithelium. Adv. Pharm. Bull. 2, 173-177. Noda, K., Nishiwaki, Y., Kawahara, M., Negoro, S., Sugiura, T, Yokoyama, A., Fukuoka M., Mori, K., Watanab, K., Tamura, T., Yamamoto, S., Saijo, N., 2002. Irinotecan plus cisplatin compared with etoposide plus cisplatin for extensive small-cell lung cancer. N. Engl. J. Med. 346, 85-91. Omar, HA, Mohamed, W.R., Arafa, E.A., Shehata, B.A., El Sherbiny, G.A., Arab, H.H., Elgendy, A.A.M., 2016. Hesperidin alleviates cisplatin-induced hepatotoxicity in rats without inhibiting its antitumor activity. Pharmacol. Rep. 68, 349-356. Paglia, D.E., Valentine, W.N., 1967. Studies on the quantitative and qualitative characterization of erythrocyte glutathione peroxidase. Lab. Clin. Med. 70, 158-169. Peterson, D.M., 2001. Oat antioxidants. J. Cereal Sci. 33, 115-129. Poirier, M.C., Reed, E., Litterst, C.L., Katz, D., Gupta-Burt, S., 1992. Persistence of platinum-ammine-DNA adducts in gonads and kidneys of rats and multiple tissues from cancer patients. Cancer Res. 52, 149-153. Purena, R., Seth, R., Bhatt, R., 2018. Protective role of Emblica officinalis hydro ethanolic leaf extract in cisplatin induced nephrotoxicity in Rats. Toxicol. Rep. 5, 270-277. Rashidi, I., Movahedin, M., Tiraihi, T., 2004. The effects of pentoxifylline on mouse epididymal sperm parameters, fertilization and cleavage rates after short time preservation. Iran J. Reprod. Med. 2, 51-57. Reddy, K.P., Madhu, P., Reddy, P.S., 2016. Protective effects of resveratrol against cisplatin-induced testicular and epididymal toxicity in rats. Food Chem. Toxicol. 91, 65-72. Salem E.A., Salem N.A., Maarouf A.M., Serefoglu E.C., Hellstrom W.J., 2012. Selenium and lycopene attenuate cisplatin-induced testicular toxicity associated with oxidative stress in Wistar rats. Urology 79, 1181-1186. Said Salem, N.I., Noshy, M.M., Said, A.A., 2017. Modulatory effect of curcumin against genotoxicity and oxidative stress induced by cisplatin and methotrexate in male mice. Food Chem. Toxicol. 105, 370-376. Seed, J., Chapi, R.E., Clegg E.D., Dostal, L.A., Foote, R.E., Hurtt, M.E., Klinefelter, G.R., Makris, S.L., Perreault, S.D., Schrader, S., Seyler, D., Sprando, R., Treinen, K.A., Rao Veeramachaneni, D.N., Wise, L.D., 1996. Methods for assessing sperm motility, morphology, and counts in the rat, rabbit, and dog: a consensus report. Reprod. Toxicol. 10, 237-244. Sherif, I.O., Abdel-Aziz, A., Sarhan, O.M., 2014. Cisplatin-induced testicular toxicity in rats: the protective effect of arjunolic acid. J. Biochem. Mol. Toxicol. 28, 515-521. Shipley, W.U., Prout, G.R., Einstein, A.B., Coombs, L.J., Wajsman, Z., Soloway, M.S., Englander, L., Barton, B.A., Hafermann, M.D., 1987. Treatment of invasive bladder cancer by cisplatin and radiation in patients unsuited for surgery. JAMA. 258, 931-935. Singh, A., Arvinda, S., Singh, S., Suri, J., Koul, S., Mondhe, D.M., Singh, G., Vishwakarma, R., 2017a. IN0523 (Urs-12-ene-3α,24β-diol) a plant based derivative of boswellic acid protect Cisplatin induced urogenital toxicity. Toxicol. Appl. Pharmacol. 318, 8-15. Singh, I., Goyal, Y., Ranawat, P., 2017b. Potential chemoprotective role of resveratrol against cisplatin induced testicular damage in mice. Chem. Biol. Interact. 273, 200-211. Tejada, R.I., Mitchell, J.C., Norman, A., Marik, J.J., Friedman, S., 1984. A test for the practical evaluation of male fertility by acridine orange (AO) fluorescence. Fertil. Steril. 42, 87-91. Türk, G., Ateşşahin, A., Sönmez, M., Ceribasi, A.O., Yuce, A., 2008. Improvement of cisplatin-induced injuries to sperm quality, the oxidant-antioxidant system, and the histologic structure of the rat testis by ellagic acid. Fertil. Steril. 89, 1474-1481. Varghese, A.C., Fischer-Hammadeh, C., Hammadeh, M.E., 2011. Acridine Orange Test for Assessment of Human Sperm DNA Integrity, in: Zini, A., Agarwal, A. (Eds.), Sperm Chromatin. Springer, New York, pp. 189-199. Vernet P., Aitken R.J., Drevet J.R., 2004. Antioxidant strategies in the epididymis. Mol. Cell Endocrinol. 216, 31-39. Waseem, M., Bhardwaj, M., Tabassum, H., Raisuddin, S., Parvez, S., 2015. Cisplatin hepatotoxicity mediated by mitochondrial stress. Drug Chem. Toxicol. 38, 452-459. Xu, C., Lv, J., You, S., Zhao, Q., Chen, X., Hu, X., 2013. Supplementation with oat protein ameliorates exercise-induced fatigue in mice. Food Funct. 4, 303-309. Yousef, M.I., Hussien, H.M., 2015. Cisplatin-induced renal toxicity via tumor necrosis factor-a, interleukin 6, tumor suppressor P53, DNA damage, xanthine oxidase, histological changes, oxidative stress and nitric oxide in rats: protective effect of ginseng, Food Chem. Toxicol. 78, 17-25. Zhang, X., Yamamoto, N., Soramoto, S., Takenaka, I., 2001. Cisplatin-induced germ cell apoptosis in mouse testes. Arch. Androl. 46, 43-49. |