|
|
Journal of Advanced Veterinary Research Volume 9, Issue 4, 2019, Pages: 134-143 www.advetresearch.com |
|
|
Pelvic Urethra and its Associated Glands in Donkey (Equus asinus): Histological and Histochemical Findings with Special Reference to their Seasonal Variations |
|
|
|
Alaa S. Abou-Elhamd1,2*, Yousria A. Abdel Rahman1, Aziza A. Selim1 |
|
|
|
1Department of Anatomy, Histology and Embryology, Faculty of Veterinary Medicine, Assiut University, Egypt. 2Department of Respiratory care, Faculty of Medical Applied Sciences, Jazan University, KSA. |
|
|
|
Received: 21 August 2019; Accepted: 22 September 2019 (*Corresponding author: alaa88@yahoo.com) |
|
|
|
Abstract |
|
|
|
The reproductive ability of male animal is dependent to a great extent on the effective functions of the genital glands. The present study was carried on the pelvic urethra of 32 sexually mature male donkeys. 5µm sections were prepared from the samples and stained with different stains to show the different structures of the pelvic urethra. Scanning electron microscopic studies were performed on the lumen of the pelvic urethra to show the different shape of the urethral gland opening on the surface layer of the lamina epithelialis of the pelvic urethra. The pelvic urethra of donkey is formed of prostatic and membranous parts. The lamina epithelialis of the pelvic urethra varied at its different regions. The urethral glands were observed along the entire length of the pelvic urethra within the lamina propria-submucosa. They were mostly of the branched tubulo-alveolar glands lined by high cuboidal or pyramidal-shaped epithelial cells. The activity of the urethral glands in donkey varied throughout the year. It was more pronounced during spring, which was manifested by increased epithelial height, decreased nuclear/cell ratio, decreasing interstitial connective tissue/glandular tissue ratio and increased cellular secretory activity. This activity decreased gradually during the summer and autumn to reach its minimal level during winter. |
|
|
|
Keywords: Donkey; Glands of Littrè; Pelvic urethra; Seasonal variations |
|
|
|
Introduction |
|
|
|
The accessory genital glands of donkey are formed of ampulla ductus deferens, seminal vesicles, prostate, bulbourethral and urethral glands (Abou-Elhamd, 2005; Abou-Elhamd et al., 2012, 2013a,b). The main function of the urethral gland is to lubricate the urethra prior to ejaculation (Plant and Zeleznik, 2015). The lining epithelium of the pelvic urethra in bulls, bucks, rams and boars had no secretory activity (Bharadwaj and Calhoun, 1959). The lamina propria-submucosa of the male pelvic urethra formed of fibroelastic connective tissue contained smooth muscle, glandular elements and erectile tissue (Krause and Cutts, 1981; Ham and Cormack, 1987; Orlandini and Orlandini, 1989; Banks, 1993). The urethral glands (glands of Littrè) were observed along the entire length of the pelvic urethra in horses and carnivores (Trautmann and Fiebiger, 1957), stallions and cats (Eurell and Frappier, 2006) and men (Fawcett and Jensh, 1997; Junqueira et al., 1998). They were considered as a part of the disseminate prostate of ruminants and swine (Trautmann and Fiebiger, 1957; Bharadwaj and Calhounn, 1959). The secretory portions of the urethral glands of men were directly linked to the epithelial lining of the urethra, while others had excretory ducts (Junqueira et al., 1998). The histological and histochemical structure of the urethral glands in donkey is rarely discussed. In our previous report, we discussed the histomorphological variations of the ampullary and prostate glands during different seasons (Abou-Elhamd et al., 2013a,b). The aim of the present study was to document the histological and histochemical structure of the pelvic urethra in donkey with special reference to the histomorphological changes in the urethral gland during different seasons. |
|
|
|
Materials and methods |
|
The present study was carried out on 32 sexually mature apparently healthy male donkeys (Jacks) of 5 to 9 years old. The animals were anesthetized and then thoroughly bled to death by severing the common carotid artery. The jacks were dissected, eviscerated and their accessory genital glands were perfused in-situ through the right and left internal pudendal arteries with the appropriate fixatives viz., neutral buffered formalin, Bouin´s fluid (for routine histological and morphometrical examination), Carnoy´s fixative (for carbohydrate histochemistry), cold formol-calcium (for lipids) and 5% glutaraldehyde in 0.1 M phosphate buffer PH 7.3 at 4 ËšC (for scanning electron microscopy). The specimens were collected from the prostatic and membranous regions of the pelvic urethra of 5 jacks in each season. The specimens were further fixed by using the similar fixatives. The tissues were processed for paraffin embedding. 5-7 μm thick paraffin sections were stained with the following stains: Harris haematoxylin and eosin for general histological examination, Crossmon´s trichrome stain for identification of collagenous and muscle fibers, Verhoeff´s method for elastic fibers, Gomori´s reticulin method for reticular fibers, periodic acid-schiff (PAS) technique for demonstration of neutral mucopolysaccharides, alcian blue technique (pH 2.5) for acidic mucopolysaccharides with or without counter staining with haematoxylin, combined alcian blue-PAS technique for acid and neutral mucopolysaccharides and Best´s carmine method for detection of glycogen content (Suvarna et al., 2018) . The morphometrical studies were performed on the stained histological sections of the glands using Lecia Q 500 MC image analyzer. The measurements carried out on each gland of the 5 jacks in all seasons in the following manner: The height of the glandular epithelium and the nuclear/cytoplasm ratio of the principal cells of the glandular epithelium of the urethral gland calculated from fifteen random fields of the glands in the present study; the interstitial connective tissue / glandular tissue ratio of the studied glands was calculated from five random fields. All the data were presented as means ± SE, which were statistically analysed using one way ANOVA and Scheffe tests. For scanning electron microscopy, the pelvic urethra cut longitudinally and washed with physiological saline solution. Parts of its luminal surface containing the openings of the glands were obtained. The specimens were further fixed in 5% phosphate buffered glutaraldehyde for 24 h at 4ËšC). Thereafter, the specimens were post-fixed in 1% buffered osmic acid for a further 2 h., and dehydrated in alcohol followed by amyl acetate. They were dried by the critical point drying using liquid Co2 and mounted on specimen’s tubes. The specimens were sputter-coated with gold, examined and photographed at JEOL 5400 LV scanning electron microscope. All procedures of the current study had been conducted in accordance with the University guidelines for the care of experimental animals. Ethical approval was obtained from the Committee of Faculty of Veterinary Medicine, Assiut University, Egypt. |
|
|
|
Results |
|
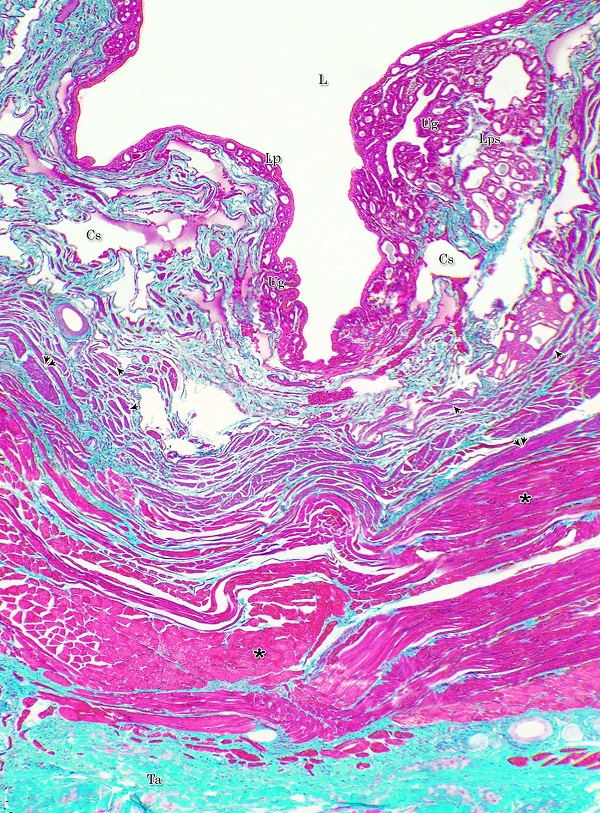
The urethral glands (Glandulae urethrales) embedded within the lamina propria-submucosa of the pelvic urethra. The latter extended from the neck of the urinary bladder to the bulb of the penis. It was divided into prostatic and membranous portions. The former extended from the neck of the urinary bladder to the caudal edge of the prostate, from which the membranous portion began and terminated at the point, where the urethra entered the bulb of the penis Histological observations Both portions of the pelvic urethra (prostatic and membranous) consisted of tunica mucosa, tunica submucosa, tunica muscularis and tunica adventitia (Fig. 1).
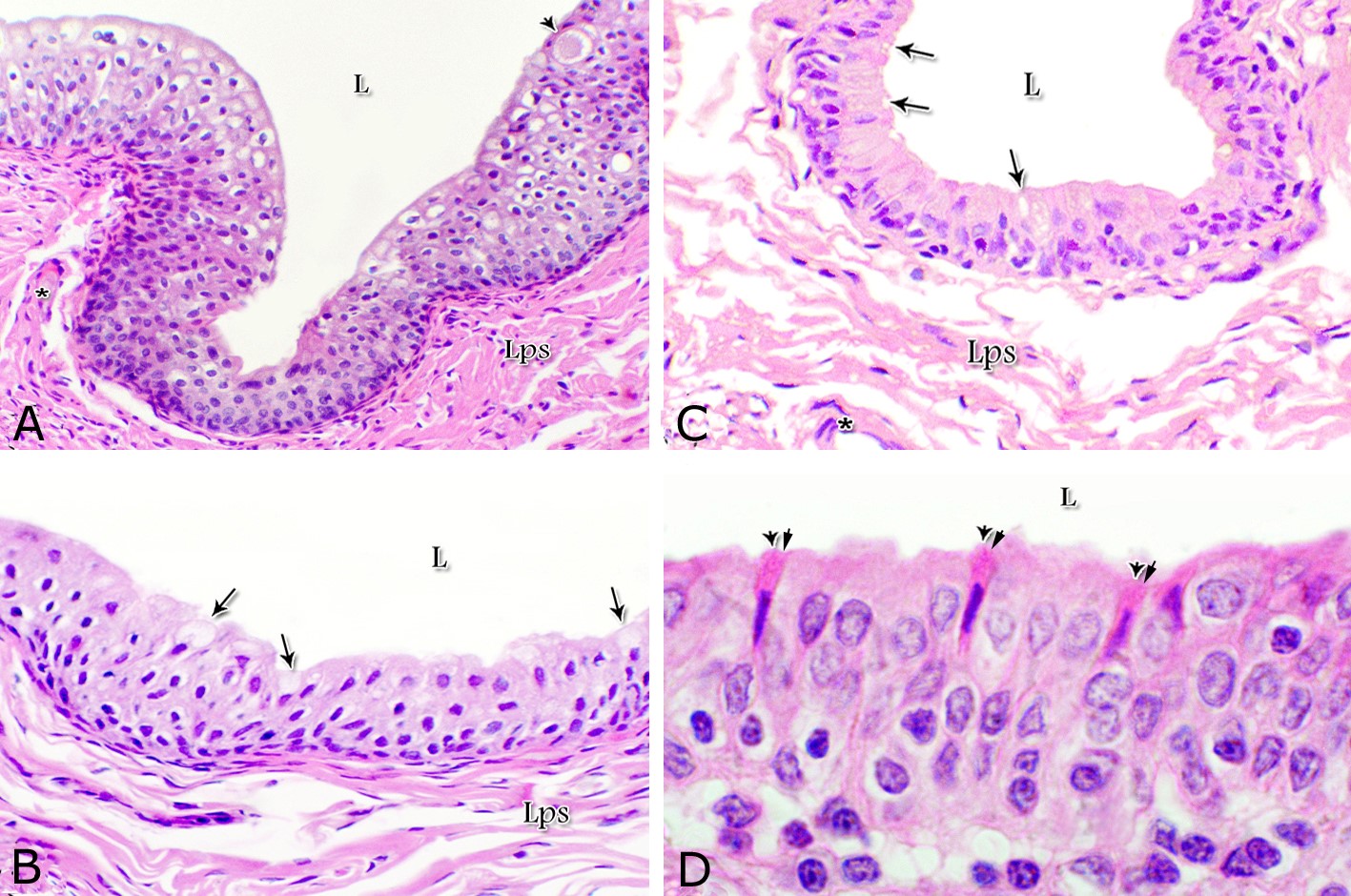
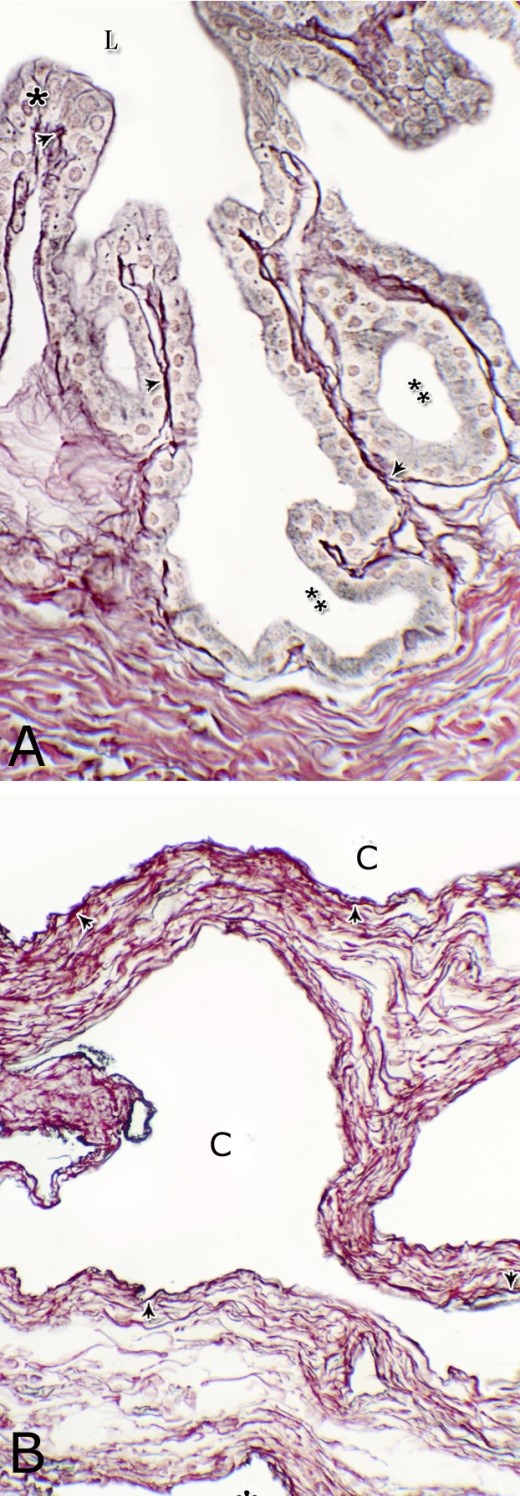
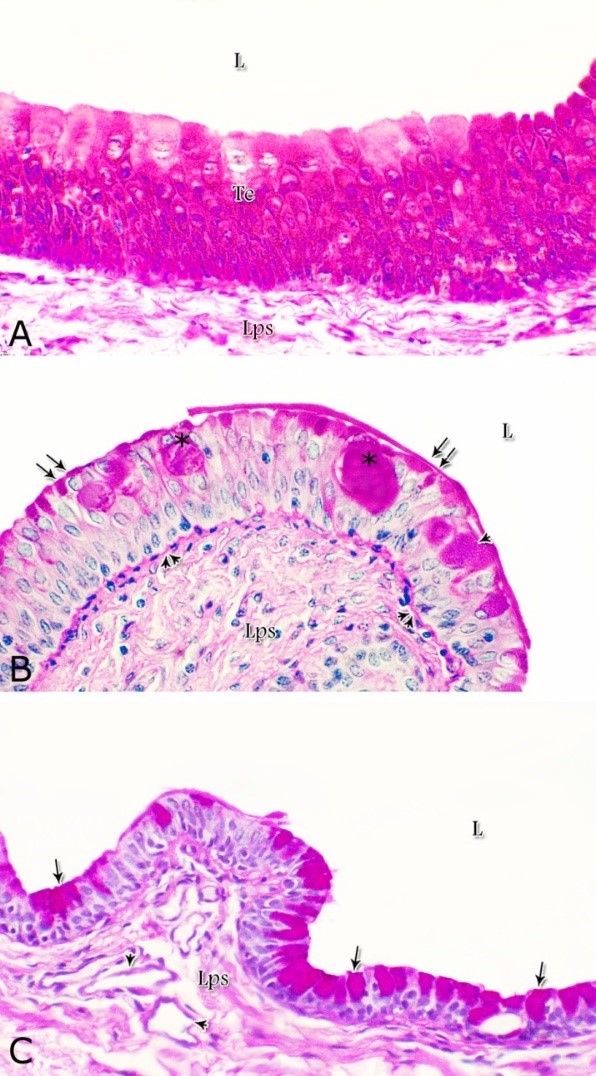
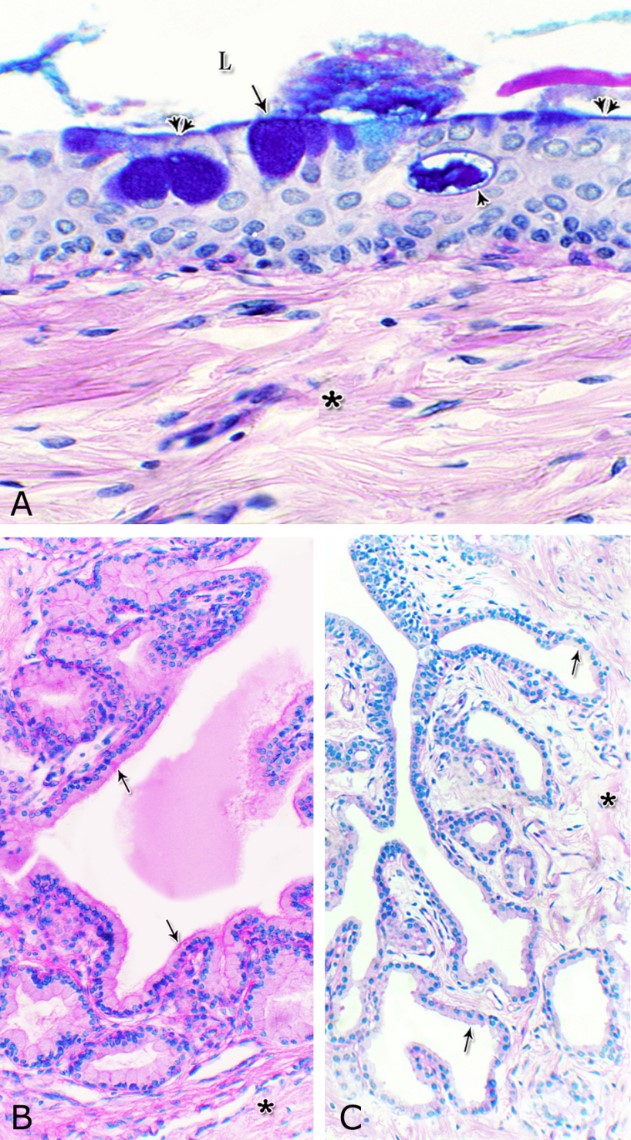
Fig. 1. Photomicrograph of the wall of the caudal part of the membranous urethra of the donkey at its ventral aspect during spring showing lamina epithelialis (Lp), lamina propria-submucosa (Lps) containing the urethral glands (Ug), inner longitudinal (arrowhead), middle circular (double arrow head) smooth muscle fiber layers, outer interwoven skeletal muscle fiber layers (asterisks) and tunica adventitia (Ta). Lumen (L), cavernous spaces (Cs). Crossmon´s trichrome stain, X50. The tunica mucosa of the pelvic urethra surrounded a wide irregular lumen, which provided with short and few variable-shaped longitudinal folds. In addition, two lateral deep invaginations (sulci) observed on either side of the Colliculus seminalis, which became shallow caudally. The lamina epithelialis of the pelvic urethra varied at its different regions. The prostatic portion was formed mainly of vacuolated transitional epithelium (Fig. 2A) with patches of stratified columnar and cuboidal one with goblet cells (Fig. 2A,B). While, at the level of the Colliculus seminalis and caudalwards, the lining epithelium consisted mainly of stratified columnar and cuboidal, which showed a gradual decrease in the height at the membranous portion. The number of the goblet cells revealed a gradual increase towards the latter portion and especially ventrally (Fig. 2C). Intra-epithelial cysts contained acidophilic secretory materials were observed in between the epithelial lining of the pelvic urethra (Fig. 2A). In addition, rod-shaped cells with darkly stained acidophilic cytoplasm and deeply stained elongated oval or flattened nuclei were observed within the stratified columnar and cuboidal epithelium (Fig. 2D).
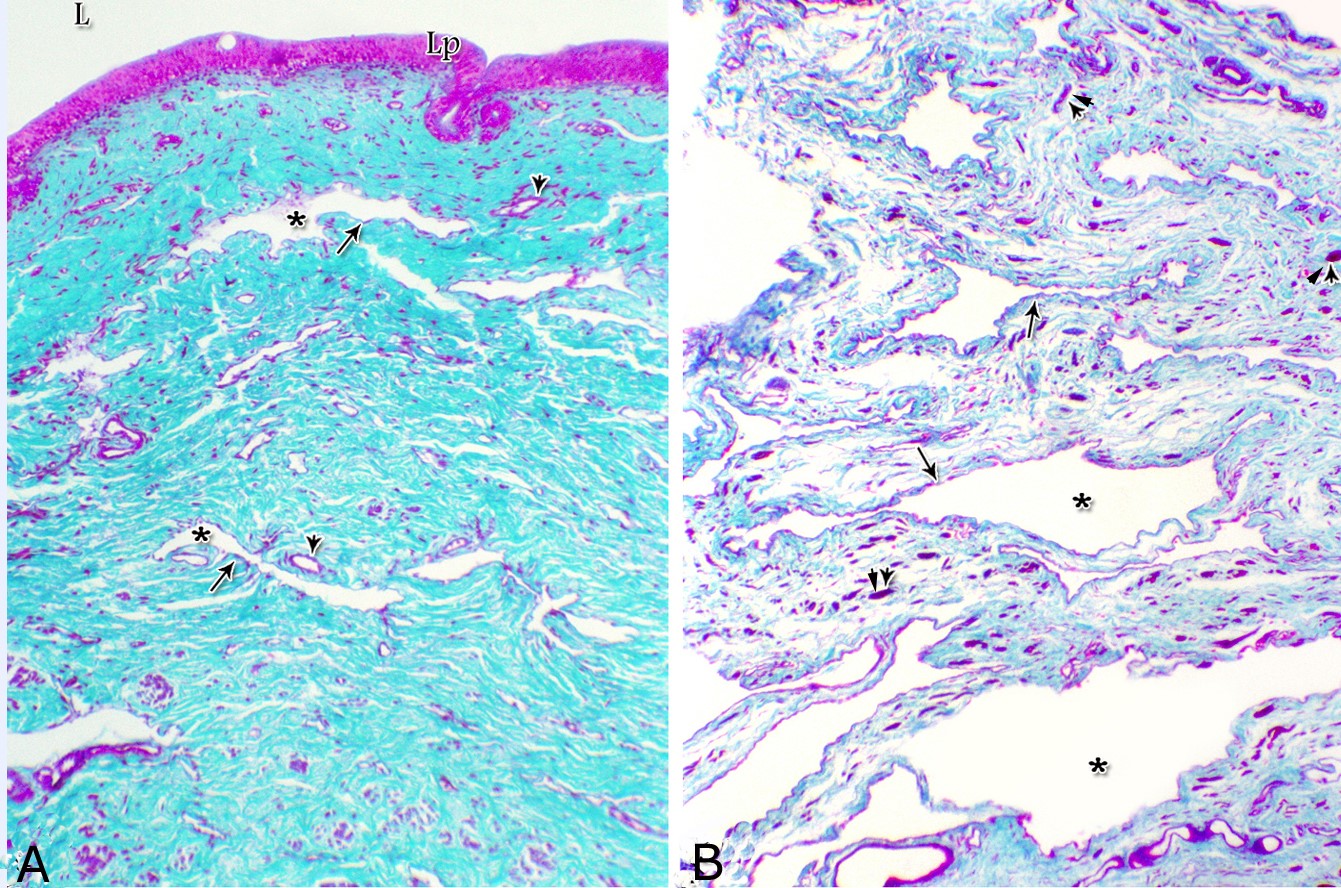
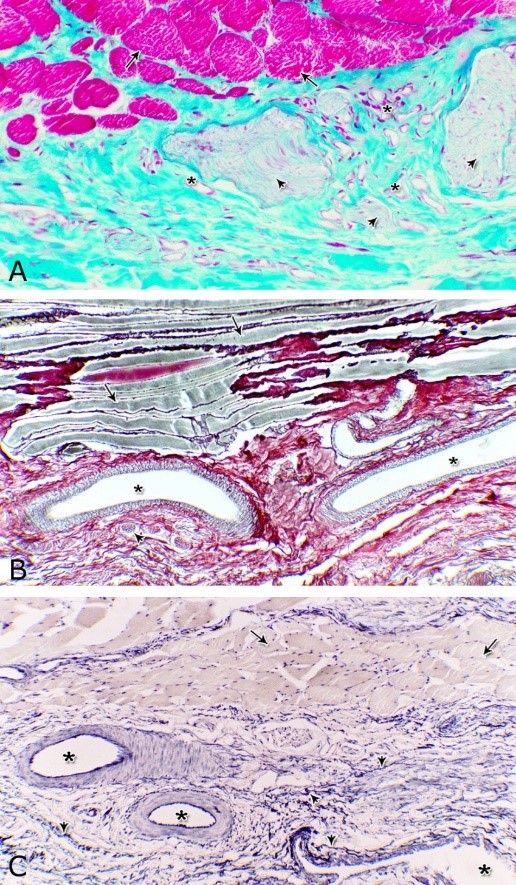
Figs. 2. Photomicrographs of the lamina epithelialis of the pelvic urethra during spring showing transitional epithelium (A) stratified columnar epithelium with goblet cells (arrows, B) of the prostatic urethra as well as that of the membranous portion (C) with numerous goblet cells in the ventral aspect. Intra-epithelial cysts containing secretory materials were observed (arrowhead, A). Rod-shaped cells (double arrowheads, D) were observed within the stratified columnar epithelium of the lamina epithelialis of the prostatic urethra of the donkey during spring. Lamina propria-submucosa (Lps) containing fibroblasts and blood vessel (asterisk), Lumen (L). Haematoxylin & eosin; A, B& C: X 400; D: X1000. The lamina propria-submucosa was composed of a highly vascular dense fibrous connective tissue layer (Figs. 3A, 4B), which appeared more elevated dorsally, where the remnants of the Mullerian ducts, the ejaculatory and the prostatic ducts (within the prostatic portion) as well as the bulbourethral glands duct openings (within the membranous portion) were located. It contained cavernous spaces and the urethral glands. The connective tissue layer of the lamina propria-submucosa consisted of coarse collagenous and abundant elastic fibers with isolated bundles of smooth muscle fibres (Fig. 3B). The collagenous fibers appeared thick and more densely packed (Figs. 1, 3A-B), while the elastic ones were more concentrated in the outer portion of this layer (Fig. 4A) especially in between the cavernous spaces (Fig. 4B). On the other hand, the reticular fibers appeared as a network supporting the epithelial lining of the urethra and its glands (Figs. 5A, B), the smooth muscle fiber bundles as well as the endothelium of the cavernous tissue. This layer also contained arterioles, venules, fibroblasts, fibrocytes and lymphocytes.
Fig. 3. Photomicrograph of the lamina epithelialis and lamina propria of the dorsal aspect of the prostatic urethra (A) and the lamina propria-submucosa of the dorsal aspect of the membranous urethra (B) during spring showing the collagenous fibers (green colour) and cavernous spaces (asterisk) lined with endothelial cells (arrows); isolated muscle bundles (double arrowheads). Blood vessels (arrowheads); lamina epithelialis (Lp); lumen (L). Crossmon´s trichrome stain. A: X50, B: X100.
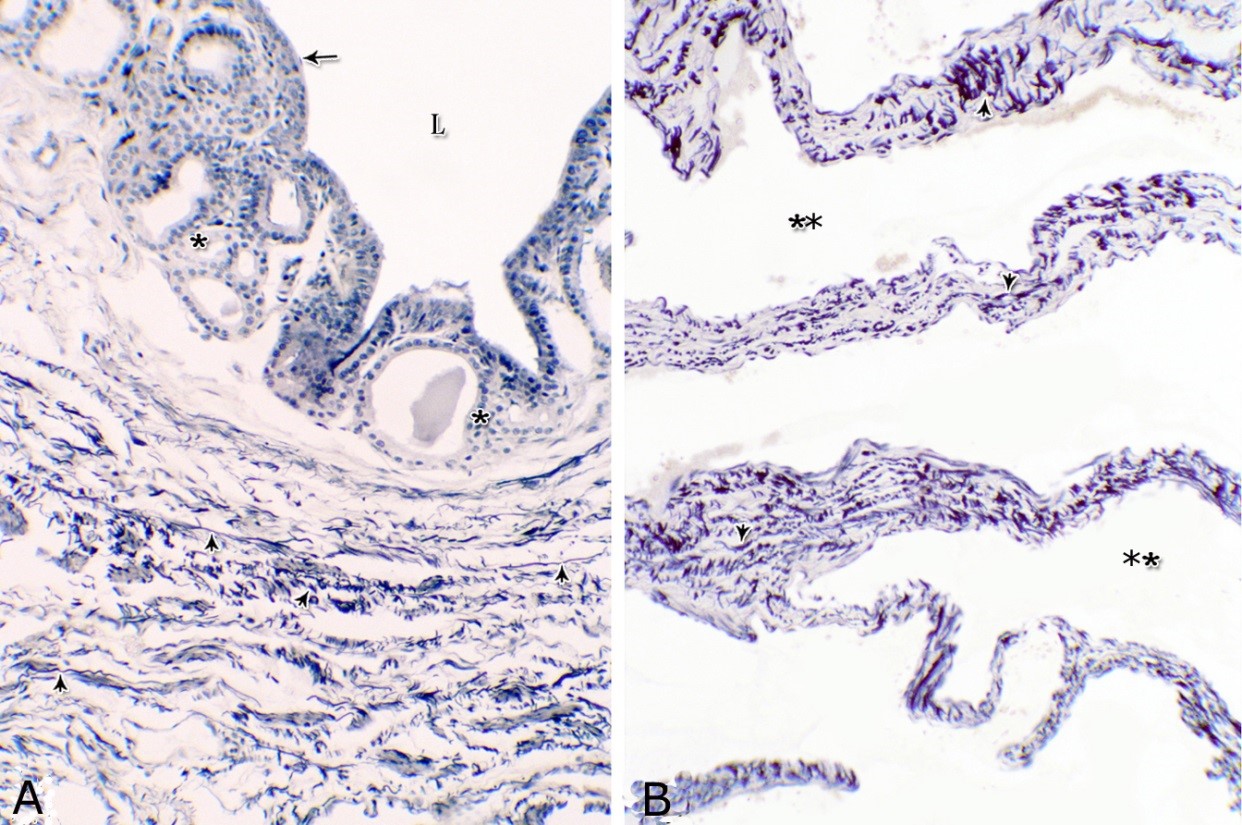
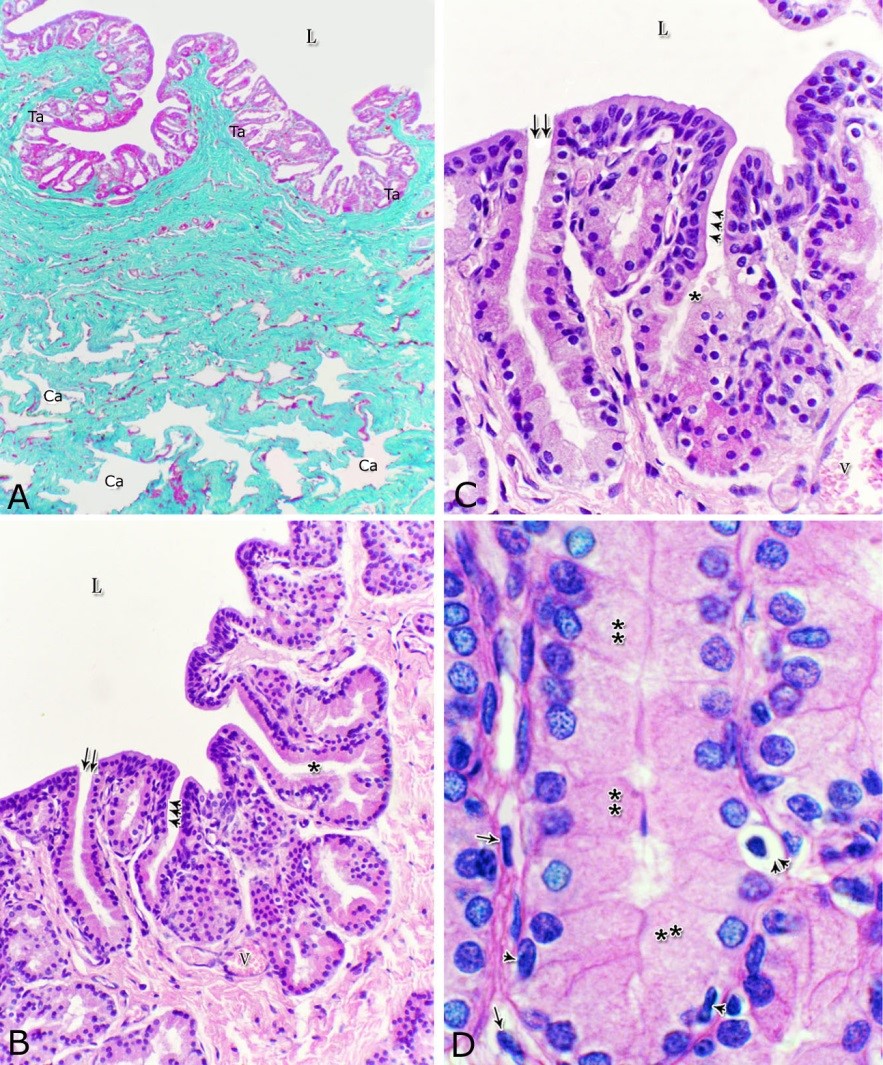
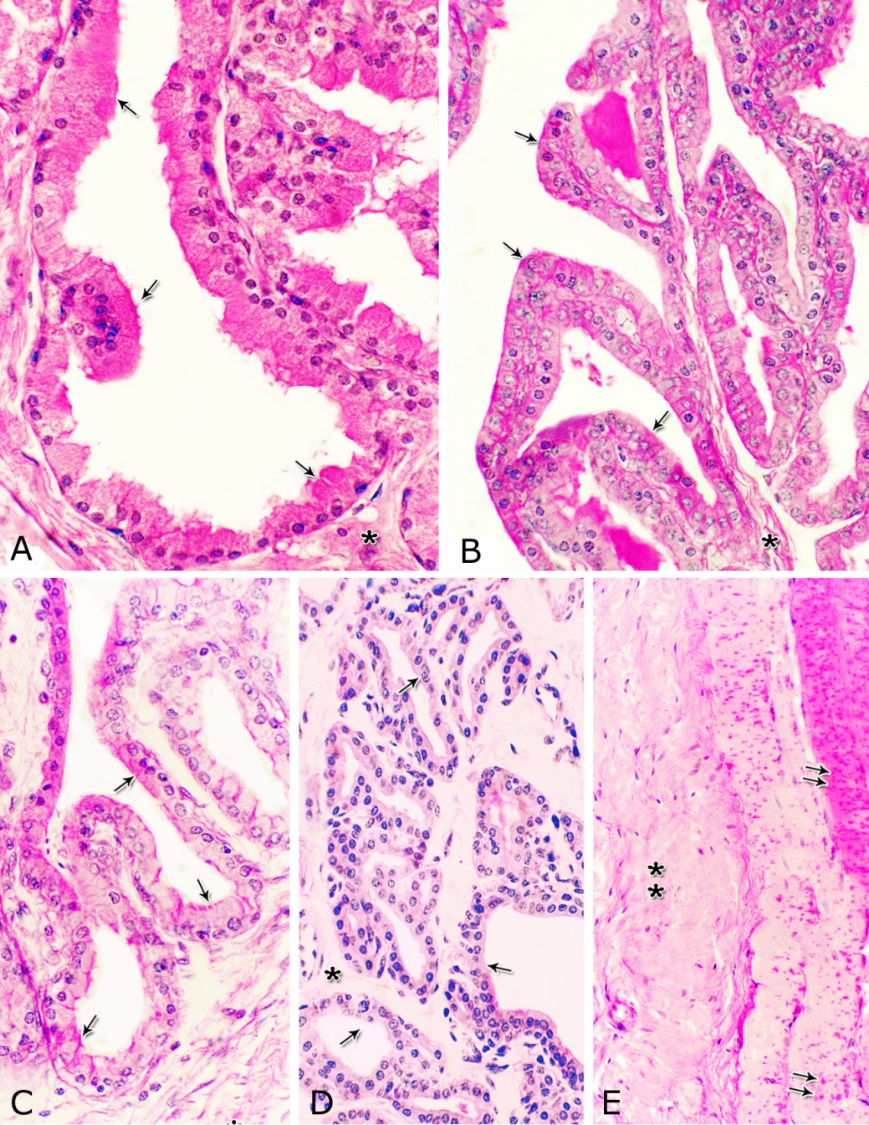
Fig. 4. Photomicrograph showing the concentration of the elastic fibers (arrowheads, A) in the outer portion of the lamina propria-submucosa in the ventral aspect of the prostatic urethra during autumn. B: Abundant elastic fibers (arrowheads) inbetween the cavernous spaces (double asterisks) in the dorsal aspect of the membranous urethra during winter. Lumen (L); lamina epithelialis (arrow); urethral glands (asterisks). Verhoff´s stain. X:200. The cavernous spaces appeared as a network of wide irregular blood spaces lined with flat endothelial cells (Figs. 3A-B, 4B) supported by reticular fibers (Fig. 5B). These blood spaces were separated from each other by thick partitions formed mainly of coarse collagenous (Figs. 3B, 5B) and abundant elastic fibers (Fig. 4B). The cavernous spaces increased in amount and diameter gradually towards the membranous portion of the pelvic urethra (Figs. 1, 3B, 4B, 5B). The urethral glands (glands of Littrè) were observed along the entire length of the pelvic urethra within the lamina propria-submucosa. They formed mostly a continuous layer at the cranial part of the membranous portion (Figs. 6A, C). They were mostly of the branched tubulo-alveolar variety, although some simple tubular glands were occasionally seen and opened directly into the lumen.
Fig. 5. Photomicrograph showing the reticular fibers network (arrowheads) supporting the surface epithelium (asterisk, A) and the urethral glands (double asterisk, A) in the membranous urethra at its ventral aspect during summer. As well as supporting the blood cavernae (C, B) at the dorsal aspect of the membranous urethra during winter (B). Collagen fibers (brown colour); lumen (L). Gomori´s stain. A: X400, B: X200. The simple tubular glands opened directly into the urethral lumen, where their lining epithelium changed gradually to cuboidal ones. The branched tubuloalveolar type opened by a short duct which lined with bi-layered epithelium, formed of superficial cuboidal and small basal cells, then become similar to the urethral epithelium close to their opening into it (Figs. 6B, C). The urethral glands (glands of Littrè) lined with principal high cuboidal or pyramidal-shaped epithelial cells with lightly stained acidophilic cytoplasm containing very fine acidophilic granules, which were more numerous during spring. Thereafter their amount decreased gradually from summer to winter. Their nuclei were nearly spherical with dispersed chromatin. The small basal cells were flat or ovoid with lightly stained vacuolated cytoplasm contained flattened or ovoid nuclei (Fig. 6D).
Fig. 6. Photomicrograph showing a continuous layer of the branched tubulo-alveolar glands (Tb) in the cranial portion of the membranous urethra (ventrally) during spring (A). Urethral glands (B) and their high magnification (C) at the ventral aspect of the membranous urethra during spring showing the simple tubular glands (double arrows) which open by a short duct direct into the urethral lumen (L). Branched tubulo-alveolar type (asterisk) and its duct lined firstly with bi-layered epithelium (triple arrowheads) which change into stratified columnar epithelium just at their opening into the urethra. D: High magnification of the urethral gland at the ventral aspect of the membranous urethra during spring showing the principal cells (double asterisks) with their granular acidophilic cytoplasm and spherical nuclei; flat (arrowheads) or ovoid (double arrowheads) basal cells with lightly stained vacuolated cytoplasm. Cavernous spaces (Ca); Fibroblasts (arrow, D); L, Lumen; collagenous fibers (green colour); V, Venules. A: Crossmon´s trichrome stain, X: 50. B, C& D: Haematoxylin & eosin. B:X 200, C: X400, D: X1000. The height of the glandular epithelium of the urethral glands of the donkey showed highly significant (P<0.01) seasonal variations. During spring, these cells reached their maximal height, where it was about 14.12±0.314 µm. A gradual decrease in the epithelial height was recorded during summer (about 11.73±0.501 µm) and autumn (about 11.56±0.353 µm). The lowest epithelial height was measured during winter where it reached about 10.65±0.186 µm (Figs. 7, 8). Fig. 7. The height of the glandular epithelium of the urethral glands of the donkey showed significant seasonal variations.
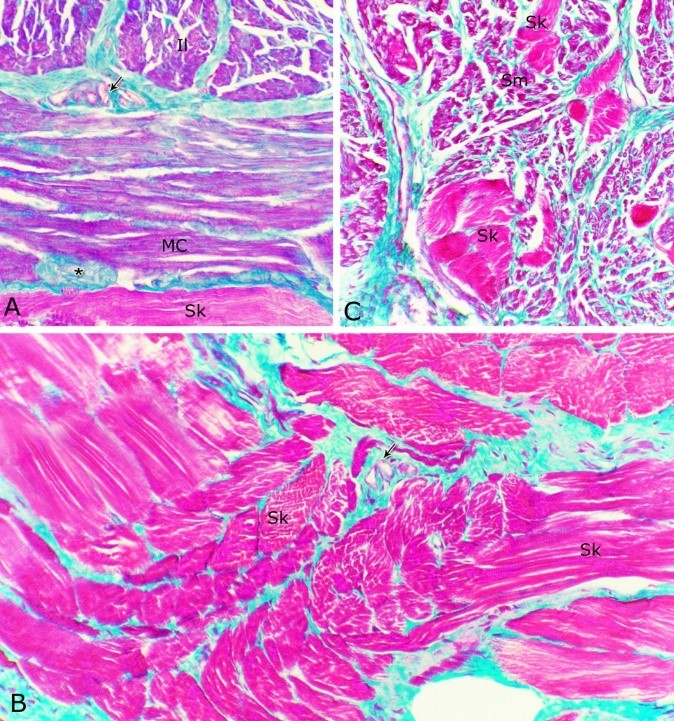
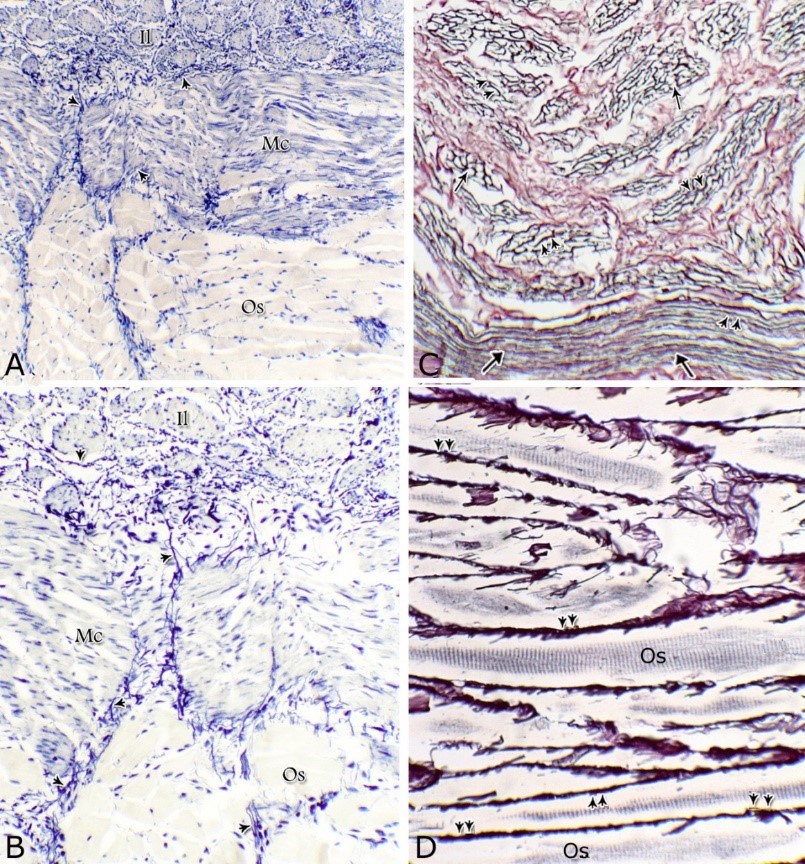
Fig. 8. Photomicrographs of the ventral aspect of the membranous urethra showing the variation in the epithelial height (arrowhead) and the amount of the interstitial connective tissue (arrow) to the glandular tissue of the urethral glands during spring (A), summer (B), autumn (C) and winter (D). Collagen fibers (green colour). Crossmon´s trichrome stain, X:400. The nuclear / cytoplasmic ratio of the glandular epithelium also showed highly significant (P<0.01) seasonal variations. The lowest ratio was observed during spring, where it was about 0.285±0.007. This ratio was increased gradually during the rest of the year. It was about 0.300±0.006 during summer and 0.310±0.005 during autumn, while the highest ratio (about 0.321±0.008) was observed during winter (Fig. 9). Fig. 9. The nuclear/ cytoplasmic ratio of the principal cells of the glandular epithelium and the interstitial connective tissue/ glandular tissue ratio of the urethral glands of the donkey showed significance seasonal variation. The interstitial connective tissue/ glandular tissue ratio of the urethral glands also revealed highly significant (P<0.01) seasonal variations. The lowest ratio (about 0.141±0.009) was observed during spring, where the amount of the connective tissue was few in comparison with that of the other season. It was observed that this ratio increased gradually to about 0.214±0.032 during summer and 0.223±0.022 during autumn. The maximal ratio (about 0.289±0.028) was observed during winter, where the amount of the interstitial connective tissue was somewhat more than that in the other seasons (Figs. 8, 9). The tunica muscularis was circumferentially surrounded the entire pelvic urethra of the donkey, except at the points, where the ducts of the prostate (at prostatic portion) and that of the bulbourethral glands (at the membranous portion) piercing it. The muscular coat was arranged into three layers of smooth and skeletal muscles (Fig. 1). The smooth muscle fibers were arranged into a thick inner longitudinal and a thin middle circular layer (Fig. 10A), while the skeletal muscle fibers were interwoven and constituted the thickest outer layer (Fig. 10B). Isolated bundles of skeletal muscle fibers were observed extending among the smooth muscle ones (Fig. 10C). The muscular coat was supported with collagenous, elastic and reticular fibers. The coarse collagenous fibers were demonstrated between the muscule bundles (Fig. 10). The elastic ones were more abundant especially among the smooth muscle fiber bundles (Figs. 11A, B). The reticular fibers formed a network enclosing each muscle fiber separately (Figs. 11C-D). The muscular coat contained also small sized arteries and veins, venules, arterioles, blood capillaries, bundles of myelinated nerve fibers, in addition to fibroblasts and fibrocytes.
Fig. 10. (A, B) Photomicrographs of the tunica muscularis of the membranous urethra at its ventral aspect during spring showing an inner longitudinal (Il) a middle circular smooth muscle layers (Mc) and outer skeletal muscle layer in an interwoven manner (Sk). (C): showing the presence of skeletal muscle fibers (Sk) among the smooth muscle bundles (Sm). Collagenous fibers (green color); bundles of unmyelinated nerve fiber (asterisk); blood vessels (arrow). Crossmon´s trichrome stain. X: 200.
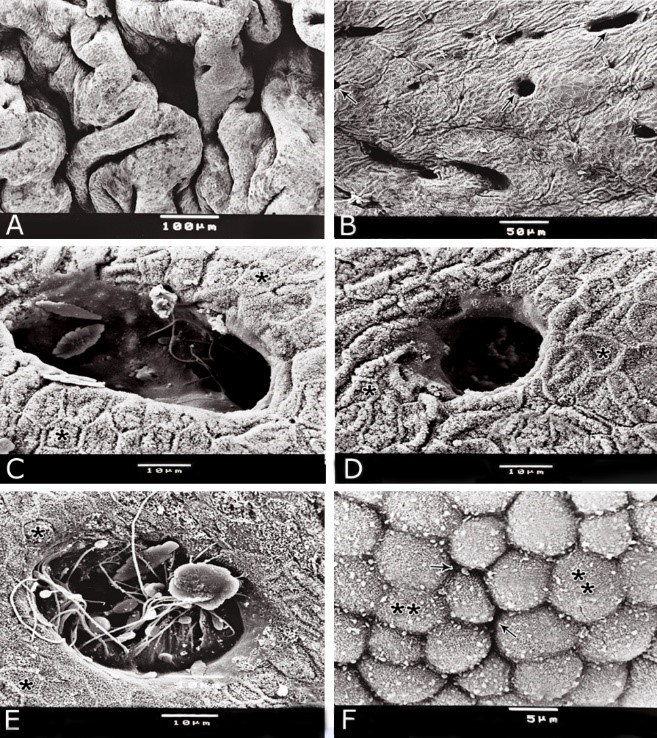
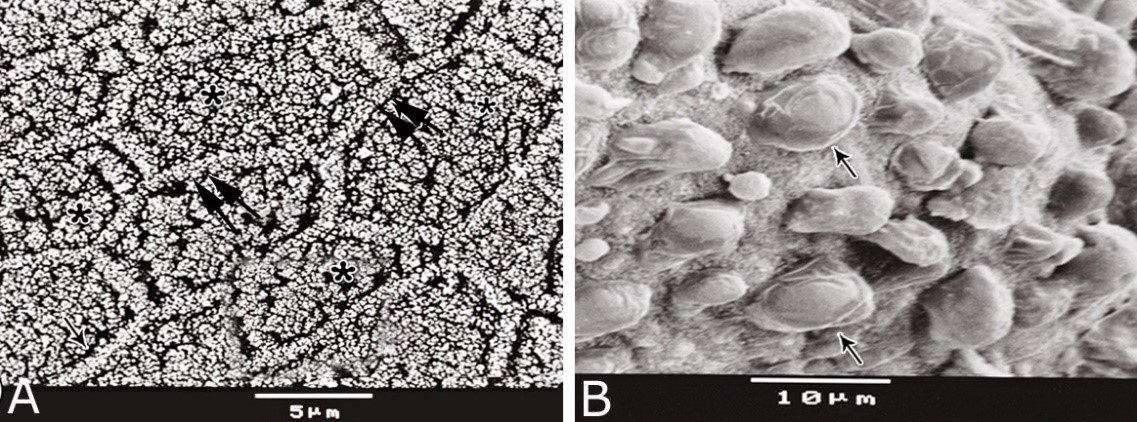
Fig. 11. Photomicrographs of a part of the tunica muscularis of the prostatic urethra at its ventral aspect (A) and high magnification (B) during spring showing the elastic fibers (arrowheads) between the inner longitudinal (Il) and middle circular (Mc) smooth muscle fibers as well as outer skeletal (Os) muscle fibers. C, D): Showing a network of reticular fibers (double arrowheads) supporting the inner longitudinal (thin arrow) and middle circular (thick arrow) smooth muscle fibers and the outer skeletal muscle fiber layer (Os) of the tunica muscularis of the prostatic urethra. Collagen fibers (brown colour). A,B: Verhoff´s stain, A: X100; B: X400. C, D: Gomori´s stain X400. The tunica adventitia covered externally the entire pelvic urethra. It was formed of dense connective tissue layer, containing coarse collagenous (Figs. 12A, B) and fine elastic fibers (Fig. 12C). Reticular fibers (Fig. 12B) were demonstrated as a network in the wall of the blood vessels. This layer contained also bundles of myelinated nerve fibers as well as ganglia, in addition to the fibroblasts and fibrocytes (Figs.12A, B). Scanning electron microscopy of the pelvic urethra of the donkey during the studied seasons showed a folded mucosal surface (Fig. 13A), with numerous randomly distributed urethral gland openings (Fig. 13B). These openings appeared mostly oval, round or ovoid (Figs. 13B- D). Some of these openings contained variable number of spermatozoa (Fig. 13C). In between the urethral gland openings, the epithelial surface of the pelvic urethra revealed regional variations. At the prostatic urethra, it was characterized by a convex apical surface of the superficial (dome-like) cells of the transitional epithelium, which were provided with numerous ill-developed microvilli and separated from each other by shallow depressions (Fig. 13F). However, at the membranous urethra, the luminal surface of the stratified columnar and cuboidal was nearly flat and characterized with somewhat hexagonal-shaped cell apexes with thick well-developed cell boundaries. Their microvilli were numerous and ill-developed (Fig. 14A). The cell apices of the goblet cells appeared packed with secretory materials causing the free border of the cells bulged towards the lumen. These goblet cells were observed within the epithelial lining of the membranous urethra and especially more numerous at its ventral aspect (Fig. 14B).
Fig. 12. Photomicrographs of the tunica adventitia at the ventral aspect of the membranous urethra during spring showing the green (A) and brown coloration of the collagenous fibers (B) and elastic fibers (arrowhead, C). Blood vessels (asterisk), skeletal muscle fibers (arrow), bundles of myelinated nerve fibers (arrowhead). A: Crossmon´s trichrome stain X: 200. B: Gomori´s stain X: 100. C: Verhoff´s stain X: 100.
Fig. 13. Scanning electron micrograph of the prostatic urethra at its dorsal aspect during spring showing folded luminal surface (A) and numerous randomly distributed urethral gland openings (arrows, B). The urethral gland opening showed oval- (C) rounded-shaped (D), which mostly contained spermatozoa (F). Hexagonal-shaped luminal cell surface (asterisks). G) High magnification scanning electron micrograph showing convex apical cell surface of the transitional epithelium (double asterisks) with numerous ill developed microvilli separated by shallow depressions (arrow).
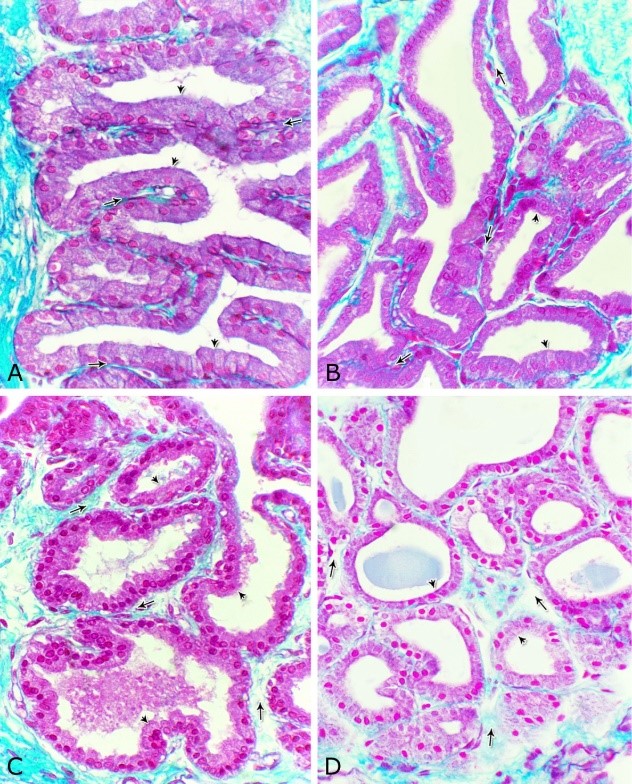
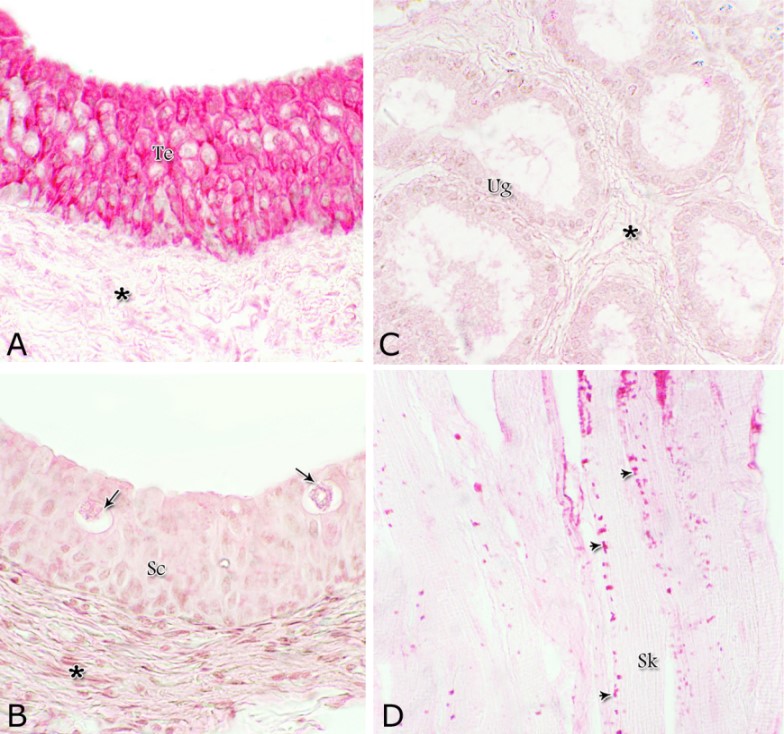
Fig. 14. Scanning electron micrograph of the membranous urethra at its dorsal aspect (A) during spring showing hexagonal-shaped cell surface of the stratified epithelium (asterisk) provided with numerous microvilli and well-developed cell boundaries (double arrows) and at the ventral aspect (B) showing numerous goblet cells packed with secretory materials (arrows). Histochemical observation Carbohydrates histochemistry Neutral mucopolysaccharides With PAS technique, the reactivity within the lamina epithelialis of both regions of the pelvic urethra of the donkey was varied. A strong PAS positive reaction was demonstrated in a granular form within the cell layers of the transitional epithelium (Fig. 15A). Within the stratified columnar and cuboidal epithelium this PAS reactivity became limited to the apical region of their superficial cells and goblet cells (Figs. 15B, C). This picture was constantly seen during all the studied seasons of the year. The intra-epithelial cysts contained also strong positive PAS substance (Fig. 15B). During spring, the epithelial lining the majority of the secretory end-pieces of the urethral glands and their luminal contents reacted strongly positive with PAS. Although, a moderate to weak PAS positive reactions were observed within few secretory end-pieces or even within the lining cells of some secretory portions (Fig. 16A).
Fig. 15. Photomicrograph showing the PAS reactivity in the epithelial lining of the pelvic urethra of donkey during spring. Strong positive PAS reactivity were observed within the cells of the transitional epithelium of the prostatic urethra (A) as well as in the apical cytoplasm of the superficial cell of the stratified columnar epithelium (double arrow) and secretory materials within the intra-epithelial cysts (asterisks) at the dorsal aspect of the prostatic urethra (B) and within the numerous goblet cells (arrow) at the ventral aspect of the membranous urethra (C). Strong PAS reaction were observed in the basement membrane (double arrowhead, B); strong to moderate PAS reactivity within the lamina propria-submucosa (Lps) as well as in the endothelium of the blood vessels (arrowhead, C). L, urethral lumen. PAS / haematoxylin stain: X400. During summer, the PAS reactivity decreased in intensity in comparison with the previous season (Fig. 16B) while during autumn, the PAS reactivity within the majority of the urethral glands ranged from moderate to weak (Fig. 16C). A further decrease in intensity of this reaction was observed during winter (Fig. 16D), where the majority of the urethral glands were weekly or even negatively reacted. In all studied seasons of the year, the connective tissue stroma forming the lamina propria-submucosa as well as those surrounding the muscular layer and that of the adventitia showed moderate PAS reaction. Strong PAS reactivity was observed in the reticular fibers supporting the muscles, the basement membrane and the wall of the blood vessels. The smooth muscle fibers showed a moderate PAS reactivity, while within the skeletal ones, this reaction had a granular appearance and ranged from strong to moderate, although some of them were negatively reacted (Fig. 16).
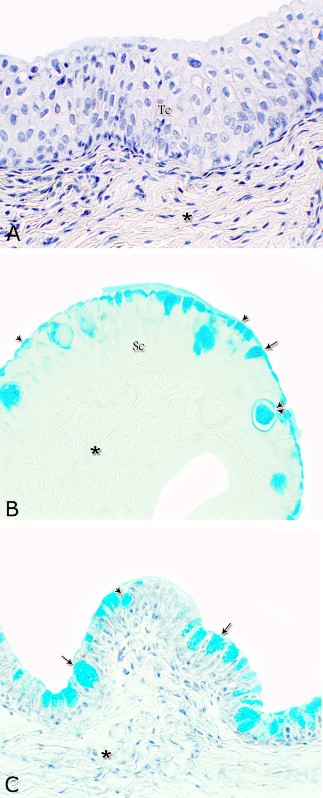
Figs. 16. Photomicrographs showing the variation in the PAS reactivity of the urethral glands (arrow) at the dorsal aspect of the membranous urethra during spring (A), summer (B), autumn (C) and winter (D). Moderately reacted connective tissue of lamina propria submucosa (asterisks). E: A variable PAS reactivity within the skeletal muscle fibers (double arrows) and moderately reacted tunica adventitia (double asterisks). PAS / haematoxylin stain. X: 400. Glycogen The cell layers of the transitional epithelium reacted strongly positive with Best′s carmine stain. This reaction appeared as a granular form (Fig. 17A). While, the stratified columnar and cuboidal epithelium, the goblet cells, the content of the intra-epithelial cysts, the wall of the blood vessels as well as the urethral glands and the connective tissue stroma were negatively reacted (Figs. 17B, C, D). However, a strong granular reactivity was observed within the muscle fibers (Fig. 17E). These pictures were constantly seen without variation in their distribution and intensity during the different season of the year.
Fig. 17. Photomicrographs at the dorsal aspect of the prostatic urethra during spring showing strong positive Best´s carmine stain reaction within the transitional epithelium (Te, A) and within the skeletal muscle fibers (Sk) at the ventral aspect of the membranous urethra (arrowheads, A). Negative Best´s carmine reaction was observed within the stratified columnar epithelium (Sc, B), intra-epithelial cysts (arrows, B) and the urethral glands (Ug, C) lamina propria-submucosa (asterisk, A). Best´s carmine stain: X400. Acid mucopolysaccharides All layers forming the pelvic urethra of the donkey revealed nearly the same picture of alcian blue during the studied seasons of the year. The transitional epithelium was negatively reacted. While, the apical borders of the superficial cell layer of the stratified columnar and cuboidal epithelium, goblet cells and the secretory material within the intra-epithelial cysts revealed strong alcian blue positive reaction (Figs. 18). The urethral glands, the connective tissue of the lamina propria-submucosa and in between the bundles of the muscular coat as well as tunica adventitia were negatively reacted for alcian blue during the four studied seasons of the year (Not shown). With alcian blue-PAS combination, the apical cytoplasm of the superficial cell layer of the stratified columnar and cuboidal epithelium, the goblet cells and secretory materials inside the intra-epihelial cysts showed a mixed picture for both stains (Fig. 19A). While, the transitional epithelium of the prostatic portion, the epithelial lining of the urethral glands, the connective tissue of the lamina propria-submucosa and those of the muscular coat and tunica adventitia as well as the muscle fibers revealed no differences beyond that recorded after PAS staining during the studied seasons of the year (Figs. 19B, C).
Fig. 18. Photomicrographs showing the alcian blue reaction within the lining epithelium of the pelvic urethra during spring. Negative reaction was observed within the transitional epithelium (Te, A) as well as the lamina propria –submucosa (asterisk) at the dorsal aspect of the prostatic urethra. Strong alcian blue positive reaction were observed within the apical cytoplasm (arrowhead) of the superficial cells of the stratified columnar epithelium (Sc, A), goblet cells (arrow) and intra-epithelial cysts (double arrowhead) at the ventral aspect of the prostatic urethra. A & C: Alcian blue / haematoxylin, B: Alcian blue: X400.
Fig. 19. Photomicrograph showing a mixed alcian blue / PAS staining at the apical cytoplasm of the stratified cuboidal epithelium (double arrowhead), goblet cells (arrow) and in the secretory materials inside the intra-epithelial cyst (arrowhead) at membranous portion of the pelvic urethra during spring. The urethral glands during spring (B) and winter (C) after alcian blue / PAS staining revealed no difference beyond that recorded after PAS staining. Moderately PAS reacted connective tissue lamina propria-submucosa (asterisk), the glandular epithelium (arrow), urethral lumen (L), Alcian blue/ PAS/ hematoxylin stain: A: X400, B,C) X200. |
|
|
|
Discussion |
|
The present study revealed that the pelvic urethra of the donkey was formed of two portions; prostatic and membranous. Each one consisted of tunica mucosa, tunica submucosa, tunica muscularis and tunica adventitia. The same results were reported in men (Krause and Cutts, 1981; Lesson and Lesson, 1981; Cormack, 2001) and domestic animals (Banks, 1993; Eurell and Frappier, 2006). Similarly to that mentioned in male urethra in men (Alm and Colleen, 1982) and domestic animals (Eurell and Frappier, 2006) the mucosa was longitudinally folded. The lamina epithelialis of the cranial part of the pelvic urethra of the donkey was a mixture of vacuolated transitional and stratified columnar epithelium that continued caudally as stratified columnar and cuboidal one. However, Bharadwaj and Calhoun (1959) in horses, cattle and boars; (Kainer et al., 1969) in bulls and (Miraglia et al., 1970) in common marmoset mentioned that, the pelvic urethra was lined only by transitional vacuolated epithelium. The present result was coincided with that of Lesson and Lesson (1981) and Orlandini and Orlandini (1989) in men who said that that the lining epithelium of the pelvic urethra of the donkey contained goblet cells, which being numerous caudally. The main function of the goblet cells may be to lubricate the urethra prior to ejaculation. Intra-epithelial cysts contained acidophilic secretory materials were also encountered within the lining epithelium of the pelvic urethra of the donkey. These cysts were quite frequent in the urethral epithelium of men and domestic animals (Maximow and Bloom, 1957; Bharadwaj and Calhoun, 1959). Also, rod-shaped cells with darkly stained cytoplasm were demonstrated in the lining epithelium of the pelvic urethra of the donkey. These cells were similar to narrow cells observed in the urethral epithelium of most domestic animals (Bharadwaj and Calhoun, 1959). The latter authors added that fibroblast-like cells were seen extending from the lamina propria-submucosa into the urethral epithelium of cattle and horses. The exact nature and function of these cells could not be established. It could be suggested that, these rod-shaped cells probably representing an apoptotic form of cell death. Similar to that observed in the pelvic urethra of camels (Ali et al., 1978) and domestic animals (Eurell and Frappier, 2006), the lamina propria-submucosa was formed of dense connective tissue, contained cavernous spaces and urethral glands. It consisted of coarse collagenous, numerous elastic and reticular fibers as well as isolated bundles of smooth muscle fibers. However, in men, the propria-submucosa of the pelvic urethra was formed of highly vascular loose connective tissue rich in elastic fibers (Lesson and Lesson, 1981; Orlandini and Orlandini, 1989). The contraction of these muscles leads to the evacuation of the urethral glands into the urethral lumen. The urethral glands (glands of Littrè) were observed along the entire length of the pelvic urethra of the donkey. Similar findings were also reported by Trautmann and Fiebiger (1957); Fawcett and Jensh (1997); Eurell and Frappier (2006) and Mescher, (2016) in the pelvic urethra of horses, carnivores, stallions and men, respectively. While, Kainer et al. (1969) stated that the urethral glands (glands of Littrè) were not observed in bulls. The presence of the urethral glands along the entire length of the pelvic urethra was probably to compensate the absence of the disseminate part in the donkey. In the same trend, the more numerously observed urethral glands at the cranial part of the membranous urethra was also to compensate the absence of secretory substances given by the prostate and bulbourethral glands at this region. The urethral glands of the donkey were mostly of the branched tubulo-alveolar; although some simple tubular glands were occasionally seen. In camels (Ali et al., 1978; Mosallam, 1981; Badawy et al., 1982) as well as stallions and cats (Banks, 1993; Mescher, 2016) the secretory units were tubular type. The present study revealed that the secretory units of the urethral glands were lined with high cuboidal or pyramidal-shaped cells and basal flat or ovoid vacuolated ones, which formed a discontinuous layer. The glandular epithelium had neither protoplasmic protrusion nor brush borders, so the mode of secretion may be merocrine. The urethral glands showed different seasonal activities. They showed maximal activity during spring and minimal activity during winter; during summer and autumn, the activity of the gland was fluctuated in between those of spring and winter. McDonald and Pineda (1989) observed that, the behavioral response in stallions is more intense and pronounced during spring and summer (the highest androgen levels) and become lowest during fall and winter (Hafez and Hafez, 2000). Also Gastal et al. (1996) stated that the libido in male donkey was more intense in spring and summer than in autumn and winter. From the concept of hormonal control and in the same trend, season is governed by secretion of melatonin in response to day light (Davies Morel, 1999). Melatonin is secreted during the hours of darkness and hence increases with shortening days that are as winter approaches (Thompson et al., 1977; Burns et al., 1982; Clay et al., 1987). This was coincided with our finding where in winter; the least activity of the urethral glands was detected. This was reflected by decreasing the epithelial height, increasing nuclear / cell ratio, increasing interstitial connective tissue / glandular tissue ratio and decreasing the cellular activity. Seasonal variations were also observed in the urethral glands of camels (Mosallam, 1981). Morphometric analysis of acinar cells of the urethral glands of mice showed that the mean volume of nuclei, cytoplasm, secretory granules, vacuoles and mitochondria were significantly reduced in castrated mice in comparison with either normal or testosterone-treated castrated mice (Parr et al., 1993). They mentioned that these glands were testosterone-dependent organs. Scanning electron microscopy of the pelvic urethra of the donkey showed a folded mucosal surface with numerous urethral gland openings. In the prostatic urethra, the superficial cells were characterized by convex apices (dome-like cells) with ill-developed micovilli. However, in the membranous part, the surface cells were hexagonal with well-developed cell boundaries and ill developed microvilli. These results were nearly similar to those observed by (Alm and Colleen, 1982; Orlandini et al., 1987; Orlandini and Orlandini, 1989) in men urethra. The scanning electron microscopy confirmed the light microscopical findings as the prostatic portion was lined with transitional epithelium, while the membranous one was stratified columnar and cuboidal in type. The lumina of some excretory ducts contained sperms. This may be due to the influx of sperms into the gland ducts during their passage through the urethra. The present study showed that the cytoplasm of the transitional epithelium lining of the pelvic urethra of the donkey was vacuolated. These vacuoles showed strong PAS and Best´s carmine stains, indicating, that the urethral epithelium contained abundant amount of glycogenic substance. This result was similar to that observed by (Barbour, 1981), who stated that the glycogen was a feature of the capping cells of the urethral transitional epithelium of hairy-nosed wombats. Glycogen in the urethral epithelium may be fermented to lactic acid by certain acid-forming bacteria, which is considered as a local defense mechanism by creating a low PH zone leading to kill of some infectious bacteria (Blandau, 1983) or contrasting the entrance of pathogenic microorganism (Orlandini and Orlandini, 1989). The abundance of glycogen in the surface coat of the lining epithelium of the urethral lumen implies hydrophilic properties (Luft, 1976). In the present work, the lining epithelium of the secretory end-pieces of the urethral glands and their contents reacted strongly positive with PAS during spring. The intensity of the reaction decreased gradually during the other seasons. The glandular epithelium was negatively reacted with alcian blue and Best´s carmine, indicating the presence of neutral mucopolysaccharides only. Similar reactivity and seasonal variations with PAS reactions were observed in the urethral glands of red deer, in which they reacted strongly during the rut season, but few of them reacted positively in the pre-rut period (Aughey, 1969). |
|
|
|
Conclusion |
|
The urethral glands of the donkey were active all over the year, but this activity was more pronounced during spring which was manifested by increasing epithelial height, decreasing nuclear / cell ratio, decreasing interstitial connective tissue / glandular tissue ratio and increasing the cellular secretory activity. This activity decreased gradually during summer and autumn and reached its minimal level during winter. |
|
|
|
Conflict of Interests |
|
|
|
The authors declare no conflict of interest. |
|
|
|
References |
|
|
|
Maximow, A.A., Bloom, W., 1957. A Textbook of Histology. W. B. Saunders Co., New York, N.Y. |
|
|