|
|
Journal of Advanced Veterinary Research Volume 9, Issue 4, 2019, Pages: 178-186 www.advetresearch.com |
|
|
Silver Nanoparticles and Sodium Hypochlorite Inhibitory Effects on Biofilm Produced by Pseudomonas aeruginosa from Poultry Farm |
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
Abd El-Moez A. Ismail, Saber A.H. Kotb, Israa M.A. Mohamed, Hosnia S. Abdel-Mohsein* |
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
Animal, Poultry Hygiene and Environ. Sanitation Dept. Faculty of Veterinary Medicine, Assiut University, Egypt. |
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
Received: 3 October 2019; Accepted: 23 October 2019 (*Corresponding author: hosnia18@aun.edu.eg) |
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
Abstract |
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
In Egypt, pseudomonas infection is one of the most important problems facing poultry production. Pseudomonas spp. is recognized as a major food spoiler and environmental contaminant. Biofilm formation by P. aeruginosa has an important role in the bacterial pathogenesis as well as persistence in the environment. The antibacterial and antibiofilm activities of AgNPs and NaOCL were evaluated against P. aeruginosa isolated from chicken farms. MIC and MBC of AgNPs against planktonic cells of P. aeruginosa were 15 and 20µg/ml, respectively. While those of NaOCL were 2200 and 2600 µg/ml, respectively. The highest inhibition percentage of biofilm formation (97.9%) was observed when P. aeruginosa treated with AgNPs (25µg/ml). While, 87.5% biofilm removal percentage was achieved after treating the established biofilm with 25 µg/ml AgNPs for 2.5 h. Moreover, NaOCL (2800 µg/ml) was able to cause 96.6% inhibition of biofilm formation and 90.3% biofilm removal after 1.5 h contact. The current study revealed that AgNPs and NaOCL were able to promote a significant reduction and removal of the mature biofilms formed by P. aeruginosa and the antibiofilm efficiency increased with the increase of its contact times with the biofilms. |
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
Keywords: Biofilm; Chicken farms; P. aeruginosa; Silver nanoparticles; Sodium hypochlorite |
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
Introduction |
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
Pseudomonas infection is one of the most important problems facing poultry production that can cause high mortality in newly hatched chicks and mass death of embryo (Kebede, 2010). The most predominant species is Pseudomonas aeruginosa. Additionally, Pseudomonas spp. is recognized as a major food spoiler and environmental contaminant (Marchand et al., 2012; Stellato et al., 2015). Pseudomonas aeruginosa is an opportunistic pathogen that can infect a broad range of hosts and can cause a wide range of infections including acute as well as chronic localized infections (Pollack, 2000; Alionte et al., 2001). The microenvironment of chickens’ house is ideal for growth of different pathogens and serves as a potential source of infection, especially for pathogens that able to survive for a longer time, which are considered as the main environment associated diseases with the difficulty in eradicating them from the chickens’ house (Shukla and Mishra, 2015). Such organisms are resistant to various antibiotic treatment and conventional disinfectants, so the control of biofilm either through prevention of its formation or by removing the already established, is considered a very important step in controlling infection. P. aeruginosa is the causative agent of several infectious diseases including endocarditis, respiratory infections, septicemia, urinary tract infections, and gastrointestinal infections and in most cases, it has been observed that the pathogenesis is dependent on its biofilm development (Wagner and Iglewski, 2008). Although less research exists about biofilms in animals, they are trusted to be involved in many diseases such as pneumonia, liver abscesses, enteritis, wound infections and mastitis infections and those infections can be caused by environmental organisms, such as P. aeruginosa (Melchior et al., 2006). P. aeruginosa pathogenesis is not related to a single virulence factor, but to interaction among different factors including biofilm formation (O'Toole and Kolter, 1998; Rocchetta et al., 1999). Bacterial biofilm has been a problem in the medical field and the presence of biofilm has been associated with various diseases. Formation of biofilms allows organisms to survive in environment, disperse to form new niches and gives them significant advantages in protection against environmental fluctuations. All kinds of animal tissues, plants and inert surfaces, can be colonized with a P. aeruginosa biofilm, thus increasing environmental persistence (Bentzmann and Plesiat, 2011). Moreover, daily cleaning with the existing products showed little effect on biofilms, as biofilms provide physical protection and reduce susceptibility to disinfectants by 10-1000-fold (Otter et al., 2015). Biofilms are difficult to control and once a biofilm has become established treatment with traditional concentrations of antimicrobials are ineffective (Hall-Stoodely and Stoodely, 2009). Studying the biofilms in the environment of poultry production and processing industries and inhibition of their growth is essential to maintain the hygienic atmosphere and ultimately ensure overall quality of poultry (Suresh et al., 2016). Moreover, the role of biofilm in veterinary field should be carefully addressed and important topics in public health, such as food safety, zoonotic disease control and animal health and welfare are highly dependent on the capability to control bacterial biofilm. There is a pressing need to develop novel alternatives that could overcome the drawbacks of the current treatment strategies. One of these alternatives could be metal-based nanoparticles (NPs) that have been utilized in several consumer products and applications due to their fast and broad antibacterial activity as well as low production costs (Fabrega et al., 2011). Silver nanoparticles (AgNPs) are now considered to be one of the most promising strategies to combat bacterial infections. The bactericidal efficiency of AgNPs has been attributed to their small size and high surface to volume ratio, and is due to the release of metal ions in solution (Morones et al., 2005; Rai et al., 2009). Furthermore, AgNPs have not been shown to cause bacterial resistance because they exert their antibacterial effects at several sites, as the bacterial wall, protein synthesis and DNA (Shrivastava et al., 2007). Smaller AgNPs can reduce more biomass and viability of biofilms, due to better penetration into the exopolysaccharide (EPS) matrix (Habash et al., 2014). Moreover, the anti-biofilm activity of AgNPs could be due to inhibition of EPS synthesis in bacteria, which limits the biofilm formation (McLaughlin-Borlace et al., 1998). There are some reports about the antimicrobial activity of AgNPs against planktonic cells of P. aeruginosa (Kora and Arunachalam, 2011; Amirulhusni et al., 2012; Singh et al., 2014). Furthermore, some studies estimated the inhibitory activity of AgNPs against P. aeruginosa and Staphylococcus biofilm (Secinti et al., 2011; de Faria et al., 2014; Palanisamy et al., 2014). However, there are very few reports estimated the efficiency of AgNPs for the removal of the established P. aeruginosa biofilm as that reported by Kalishwaralal et al. (2010) concerning biofilms formed by P. aeruginosa and S. epidermidis responsible for microbial keratitis. Therefore, the aim of the present study was to evaluate the efficiency of AgNPs to eradicate the built-up mature biofilm by P. aeruginosa isolated from poultry environment. This might highlight the hygienic significant role that AgNPs can play to control the persistence and spread of P. aeruginosa infection in both animal and poultry house, food industry plants, hospitals or any other area where pseudomonas biofilm could be exist. Furthermore, the antibacterial and antibiofilm efficiency of AgNPs was compared with those of sodium hypochlorite (NaOCL). Sodium hypochlorite is one of the most effective disinfectants against biofilms and during treatment it decomposes into sodium hydroxide and hypochlorite, which is a strong oxidizing agent that act by oxidizing the cell membrane of pathogen resulting in cell lysis and death (Hawkins and Davies, 1999; Tote et al., 2010; Rodrigues et al., 2011). |
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
Materials and methods |
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
Bacterial strains Pseudomonas aeruginosa strains were isolated from chicken farms at Assiut Governorate, Egypt. Six hundred samples were collected from 11 farms representing water, feed, litter samples, as well as water troughs, cloacal and wall swabs. Isolation of P. aeruginosa was carried out according to Corry et al. (2011). Tryptic soy broth (TSB) was used for enrichment and incubated at 37 oC for 24 h. A loopful from the enrichment broth was streaked on pseudomonas isolation agar (biolife DF4403) supplemented with 10% glycerol and incubated at 37 oC for 24 h. The greenish and bluish pigment producer colonies were selected for further identifications with Gram staining and biochemical reactions (Winn, 2006; Versalovic et al., 2011) followed by molecular confirmation by conventional PCR that was carried out in the Molecular Biology Research Unit (MBRU), Assiut University, Egypt. P. aeruginosa strain from Animal Health Research Institute, Giza, Egypt was used as a positive control during PCR analysis. PCR was performed using primer set; ECF1 and ECF2 (Lavenir et al., 2007). Patho Gene DNA/RNA extraction kit (iNtRON Biotechnology) was used for extraction process according to the manufacture’ instructions. PCR reaction was done in a total volume of 20 ul containing 10 ul PCR master mix, 1 ul of each primer and 8 ul of the extracted DNA. The thermocycler conditions included initial denaturation at 95 °C for 5 min., followed by 35 cycles of 95 °C for 45 sec., 58 °C for 45 sec and 72 °C for 1 min with final extension at 72 °C for 10 min. After amplification, 1% agarose was used for electrophoresis. P. aeruginosa strains were checked for their ability to build up biofilm using tissue culture plate method (TCP) (Coffey and Anderson, 2014). Fresh colonies of each strain were transferred to 5 ml TSB and incubated at 37 oC for 18 h. Then, a 1:100 dilution was prepared in TSB using 1% glucose as a supplement (GanjaliDashti et al., 2016) and following thorough mixing, 100 ul was transferred to each well in 96 microtiter tissue culture plates and incubated at 37 oC without shaking for 24 h. After incubation the liquid media was gently removed from each well and the wells were washed 3 times with distilled water. The wells were stained with 125 ul of crystal violet (0.1%) for 15- 30 min to confirm the presence of biofilm. Excess stain was cleaned by washing with distilled water and the plates were kept for 30 min till dryness. The microtiter plate was examined for the presence of purple ring that indicating the presence of biofilm. Efficiency of AgNPs and NaOCL against P. aeruginosa biofilm Preparation of AgNPs Stable AgNPs less than 100 nm was synthesized according to Vigneshwaran et al. (2006). Briefly, soluble starch (one gram) mixed with 100 ml of deionized water was heated till complete dissolution, after that 1 ml of a 100 mM aqueous solution of silver nitrate (AgNO3), was added and stirred well. This mixture was put into dark glass bottle and autoclaved for 5 min at 121 oC. The resulting solution had clear yellow color indicating the formation of AgNPs. The stock solution was kept away from direct sunlight at room temperature. AgNPs total concentration was estimated using Graphite Furnace Atomic Absorption (Model 210VGP) at the Faculty of Science, Assiut University, Egypt. The size of the prepared particles was analyzed with Transmission Electron Microscope (TEM) (JEOL-JEM- 100CX II) at Electron Microscopy Unit, Assiut University, Egypt. Determination of minimum inhibitory concentration (MIC) and minimum bactericidal concentration (MBC) of AgNPs and NaOCL MIC of AgNPs and NaOCL against 4 strains of P. aeruoginosa was measured by standard tube macro dilution broth method (Sharma et al., 2015). Different concentrations of AgNPs (10, 15, 20, 25, 30, 35 and 40μg/ml) and NaOCL; 5 % available chlorine (100 till 3000μg/ml) were separately added to each tube containing 5 ml TSB and finally an aliquot of bacteria culture (5×105 CFU ml-1) were separately added to these tubes. After that, the tubes were incubated at 37 ˚C for 18 h. In the control positive set, only bacterial cells were grown in absence of AgNPs and NaOCL, while tubes that contain TSB plus AgNPs or NaOCL in absence of inoculums were considered control negative set. The MIC was considered as the lowest concentration of AgNPs and NaOCL with no visible bacterial growth after incubation. To determine MBC, the lowest concentration, which give no visible turbidity, and two higher concentrations were sub-cultured into sterile petri dishes containing standard plates count agar to assure the absence of colony forming units (CFU). The petri dishes without any CFU were considered positive for the MBC. Prevention and eradication of bacterial biofilm by AgNPs and NaOCL The capability of AgNPs and NaOCL to prevent and control biofilm was determined according to Abidi et al. (2014). Four strains of P. aeruginosa were tested, and glucose supplement (1 %) was applied during bacterial growth for biofilm production. The anti-biofilm activity of AgNPs and NaOCL was evaluated during bacterial incubation; while the biofilm was being created. Briefly, fresh overnight colonies of each strain were transferred to TSB and allowed to grow at 37 oC for 18 h. Then the culture was diluted 1:100 in TSB with glucose supplement and 100 μl of diluted culture was pipetted in microtiter plate wells. After that, 100 μl of each concentration of AgNPs or NaOCL was inoculated into 3 wells and the plate was covered and incubated at 37 oC for 24 h. Each bacterial strain was treated with 4 concentrations of AgNPs (10, 15, 20, and 25 μg/ml) and 4 concentrations of NaOCL (2200, 2400, 2600 and 2800 μg/ml). Furthermore, for each strain, 3 wells of the 96-well flat bottom microtiter plate were inoculated with bacterial inoculums without treatments (positive control) and another 3 wells for negative control (treatment with TSB only). Concerning the second experiment, anti-biofilm activity of AgNPs and NaOCL was estimated after incubation; on the established biofilm. Microtiter plates were inoculated as mentioned above and incubated for biofilm production. After incubation, plates were washed with sterile water to remove planktonic cells after that200μl of each tested agent (25μg/ml AgNPs and 2800 μg/ml NaOCL) was transferred into each well, with exception of blank and positive control wells. The plates were incubated for different contact times, namely (15, 30, 45, 60, 90, 120, and 150 min.) for AgNPs and (5, 15, 30, 45, 60 and 90 min.) for NaOCL. At the end of each contact time, AgNPs and NaOCL were quenched by adding 5 g/L sodium thiosulfate (Na2S2O3) to stop their antimicrobial reaction as described in the European quality standards (NEN-EN 1276, 1997). Following the desired incubation time, the planktonic bacterial cells were removed, and the wells were washed several times. Subsequently, for biofilm staining, 125μl of 0.1% crystal violet solution was added to each well and incubated for 10 min at room temperature then the stain was removed, and plates were washed with vigorous shaking to remove all liquid. Subsequently, the plates were inverted and left to air dry. For biofilm quantification the dye was solubilized by adding 150μl of 30% acetic acid to each well of the plate and incubated for 10-15 minutes at room temperature. Then, contents of each well were thoroughly mixed and 125μl of the crystal violet-acetic acid solution was transferred from each well to a separate well of a new optically clear flat- bottom 96-well plate. Optical density (OD) of each of these 125μl samples was measured at a wavelength 545 nm. And to measure the antibiofilm efficacy; the reduction/ removal percentages were calculated using the following equation Reduction/Removal Percentage = [(C-B) - (T-B) / (C-B)] *100% Where B = absorbance of blank (no biofilm, no treatment). C = absorbance of control (biofilm, no treatment). T = absorbance of test (biofilm and treatment). Statistical analysis Statistical analysis of the obtained data was carried by using SPSS software version 17. The data was subjected to analyses of variance using the ANOVA procedure and General Linear Models Procedure (GLM procedure) of SPSS software. The results of optical densities were presented as mean and standard deviations (SD) for each variable. Significant differences between mean values were tested using Duncan's multiple range test. P-value is considered statistically significant when P < 0.05. |
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
Results |
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
P. aeruginosa from poultry farms and the ability to build up biofilm In this study, P. aeruginosa was isolated from eleven layer and broiler farms at Assiut Governorate, Egypt and 15 biochemically positive strains were tested using conventional PCR. The data illustrated in Fig. 1 and Table 1, revealed the molecular identification of P. aeruginosa. Four strains were positive for P. aeruginosa representing feed and water troughs swabs. The ability of these strains to build up biofilm was proved with tissue culture plate method. Table 1. Biochemical and molecular identification of P. aeruginosa by conventional PCR
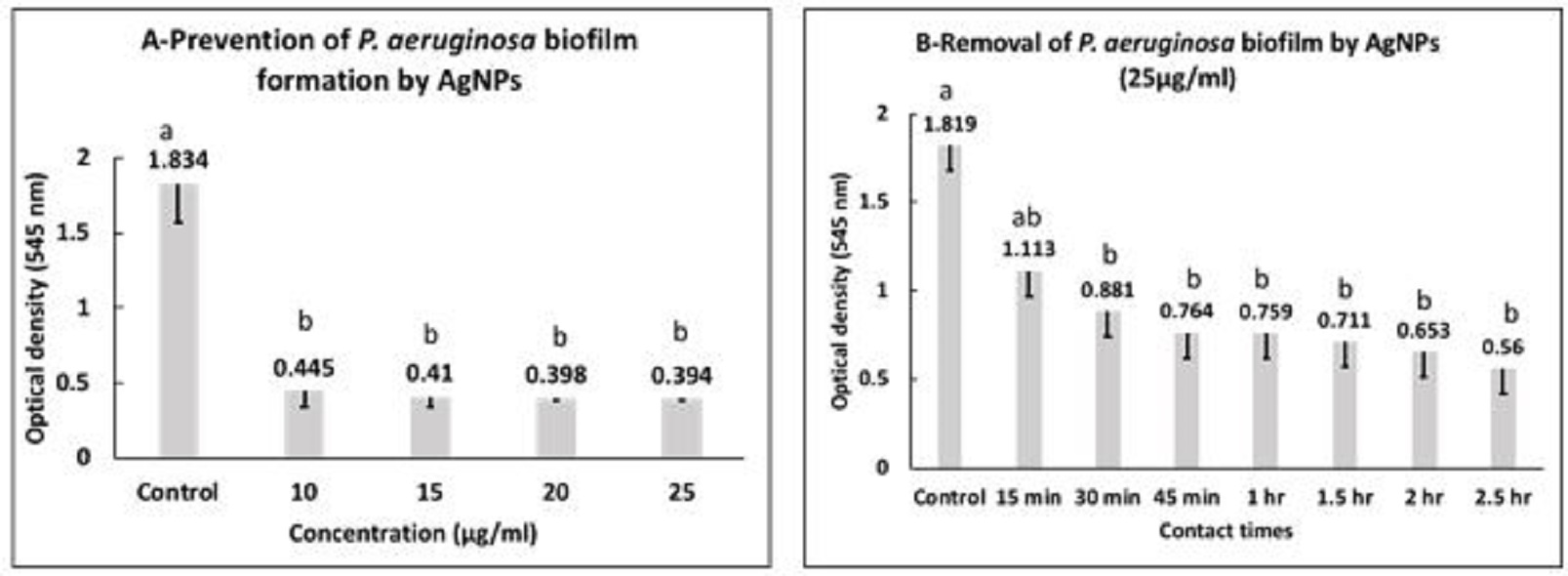
Fig. 1. Agrose gel electrophoreses of PCR products with P. aeruginosa specific primers (528 bp). M: 100 bp DNA marker, pc: positive control, nc: negative control, Lanes 8, 9, 11 and 14 are positive for P. aeruginosa. Inhibitory effect of AgNPs against P. aeruginosa AgNPs showed MIC and MBC against planktonic P. aeruginosa at 15 and 20 µg/ml, respectively. Concerning the inhibitory effect of AgNPs on biofilm build up ability of P. aeruginosa, the data presented in Fig. 2a, represents the mean values of optical densities (OD) ± standard deviation of the solubilized biofilm formed by P. aeruginosa treated with 10, 15, 20 and 25 µg/ml of AgNPs. The statistical analysis of these data showed that at all used concentrations of AgNPs the mean values of optical densities of bacterial culture was significantly reduced in all exposed P.aeruginosa when compared with the control group (P<0.05). Furthermore, there were no significant differences among the mean values of optical densities of bacterial culture exposed to different concentration of AgNPs. The highest inhibition percentages of biofilms (97.9 %) was observed when P. aeruginosa incubated with 25 µg/ml of AgNPs. The antibiofilm activity of AgNPs to control the established biofilm was evaluated by treating P.aeruginosa biofilms with AgNPs (25µg/ml) for different contact times. The data presented in Fig. 2b, showed that the mean values of OD ± SD of biofilms formed by P. aeruginosa treated with 25 µg/ml AgNPs after contact times of 15 min, 30 min, 45 min, 1 h, 1.5 h, 2 h, 2.5 h.The analysis of variance of the obtained data showed that there were significant differences between all contact times and control group (P<0.05) except at contact time of 15 min., a removal percentage of 87.6% was recorded after treating the established biofilm with 25 µg/ml AgNPs for 2.5 h.
Fig. 2. Prevention (A) and eradication (B) of P. aeruginosa biofilm by AgNPs. a,b: means with different letters are significantly different. Inhibitory effect of NaOCL against P. aeruginosa MIC and MBC of NaOCL on P. aeruginosa at 2200 and 2600 µg/ml, respectively. Fig. 3a, presented the mean values of OD ± SD of biofilms formed by P. aeruginosa treated with 2200 µg/ml, 2400 µg/ml, 2600 µg/ml and 2800 µg/ml of NaOCL. The statistical analysis of data showed that at all used concentrations the mean values of optical densities was significantly reduced when compared with the control group (P<0.05). Furthermore, there were no significant differences among the mean values of optical densities of bacterial culture subjected to all used concentration. NaOCL was able to cause inhibition percentages of 93.2% and 96.6% at concentrations 2200 and 2800 µg/ml, respectively. For control of P. aeruginosa biofilms with NaOCL (2800 µg/ml), the data presented in Fig. 3b revealed that the mean values of OD ± SD of P. aeruginosa biofilms treated with 2800 µg/ml NaOCL after different contact times. The analysis of variance of data showed that there were significant differences between all contact times and the control group (P<0.05). Moreover, there were no significant differences in between all contact times. The percentages of biofilms removal after contact times of 5 min, 1.5 h were 81.4% and 90.3%, respectively. Fig. 3. Prevention (A) and eradication (B) of P. aeruginosa biofilm by NaOCL. a,b: means with different letters are significantly different. |
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
Discussion |
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
The prevalence of P. aeruginosa in water troughs swabs and water agreed with the findings of Kumar et al. (2009), Mena and Gerba (2009) and Peix et al. (2009), who reported that P. aeruginosa was widely present in water and humid environments, which could be the initial source of infection. Furthermore, P. aeruginosa has a great capability to colonize and form strong and mature biofilms on all wet surfaces including all kinds of animal tissues, plants and inert surfaces, thus increasing its environmental persistence (Bentzmann and Plésiat, 2011; Cherif et al., 2016). P. aeruginosa has the ability to construct complex biofilm matrix molecules including polysaccharides, nucleic acids and proteins. P. aeruginosa can readily form biofilms in any environment favorable to growth, whereas other species may require specific cues such as temperature, pH or nutritional factors (O’Toole et al., 2000). Biofilms support microbial growth and have an enclose impact in veterinary field as, they are responsible for the failure of antimicrobial therapy and of sanitations in food processing plants, animal and poultry houses. Conventional antibiotics and sanitation procedures can be effective against planktonic pathogens but are often weakly effective against the bacteria in biofilm (Yarwood and Schlievert, 2003). The biofilms are responsible for causing a broad range of chronic diseases and the emergence of antibiotic resistance. Hence, it is critically important to design or screen anti-biofilm molecules that can effectively minimize and eradicate biofilm related infections (Royet al., 2017). As an opportunistic human pathogen, P. aeruginosa isolates from food products and food processing environment represent a significant risk for public health that cause great safety concern in food sectors (Cherif et al., 2016). Therefore, the research to find new compounds with high antibiofilm activity is urgently required. AgNPs have abroad antimicrobial activity spectrum against both Gram-positive and Gram-negative bacteria and the use of AgNPs is now considered as one of the most promising strategies to combat biofilm infections related to medical devices (Elliott, 2007).The recorded inhibitory concentration was lower than that reported by Amirulhusni et al. (2012), who revealed that AgNPs (size ranged from 20–30 nm)exhibited MIC and MBC of 50 and 100 μg/ml against multidrug resistant P. aeruginosa, respectively. As a result, we could achieve an efficient growth inhibition of P. aeruginosa at a low and minimum concentration of 15 µg/ml. The size of AgNPs ranged between 11.7 to 18.9 nm with spherical shape where at this size, AgNPs was able to disrupt planktonic Pseudomonas. The bactericidal effect of AgNPs is dependent on the size and shape of the particles and the specific surface area increases as the particle size decreases, allowing greater particles interaction with bacterial cells and the surrounding environment, in addition, triangular-shaped particles display more anti-bacterial activity than rods or spherical particles (Pal et al., 2007). At the lowest tested concentration (10 µg/ml) there was a 94% reduction in the biofilm bacterial mass while at the highest concentration (25 µg/ml) there was nearly a complete reduction in the biofilm formation. These results indicated that the starch-stabilized AgNPs not only exhibit potent antibacterial activity, but also impede the biofilm formation. A lower inhibition rate was revealed by Palanisamy et al. (2014), who reported that 20μg/ml AgNPs (sizes ranged from 20–30 nm) showed 56-67% inhibition rate of P. aeruginosa biofilm formation. Kalishwaralal et al. (2010) mentioned that AgNPs (mean diameter 50 nm) at a concentration of 10-100 nM prevented P. aeruginosa biofilm formation by impeding the initial step (synthesis of exopolysaccharides and bacterial adhesion to the surface) at the low concentration without affecting the cell viability while at the high concentration inhibited the growth of bacteria. Moreover, Mohanty et al. (2012) revealed that starch-stabilized nanoparticles (about 20 nm in diameter), at very low concentrations of 1–2 μM, decreased P. aeruginosa biofilm formation by 50% and 65% after 24 and 48 h treatment, respectively. Martinez-Gutierrez et al. (2013) found that AgNPs (25 nm diameter) effectively prevented the formation of P. aeruginosa biofilms and kill bacteria in established biofilm structures (4-log reduction in the bacterial cells number). Radzig et al. (2013) found that 5 -10μg/ml AgNPs (8 nm in diameter) stabilized by hydrolyzed casein peptides, strongly inhibited biofilm formation by some Gram-negative bacteria and decreased bacterial mass in P. aeruginosa biofilms. Secinti et al. (2011) found that AgNPs inhibited P. aeruginosa biofilms by 95%. During biofilm formation, biofilms develop themselves into different stages: planktonic, attachment (reversible and irreversible), maturation (microcolonies and macrocolonies) and dispersion (Monds and O’Toole, 2009). And since biofilm shows different characteristics during formation and maturation, biofilms in different stages may have different susceptibility to AgNPs. The anti-biofilm activity of AgNPs could be due to inhibition of exopolysaccharide synthesis, which limits the biofilm formation. Moreover, smaller AgNPs can reduce the biomass and viability of biofilms, due to better penetration into the EPS matrix (McLaughlin-Borlace,1998; Habash et al., 2014) suggesting that it could be used to delay biofilm formation and for the prevention of biofilm-related infections. The obtained results revealed that AgNPs was effectively eradicate the mature biofilms formed by P. aeruginosa. However, the results reported by Thuptimdang et al. (2015) revealed that P. putida mature biofilms were not susceptible to AgNPs. Several factors may explain the increased resistance of mature biofilms to antimicrobial agents; (1) bacterial cells in mature biofilms are to be in the stationary growth phase (less susceptible to antimicrobial agents), (2) cells that die in the outer layers of mature biofilms could provide nutrients for the growth of cells in deeper layers, (3) the high thickness or high amount of EPS may have a role in transport limitations of AgNPs through biofilms, (4) mature biofilms, produce not only more EPS but also different components as, curli which is a protein component used for bacterial adhesion to surfaces (Anderl et al., 2003; Ito et al., 2009; Saldaña et al., 2009). Contrary, Wong et al. (2010) have reported that the age of the biofilm does not enhance resistance to disinfectants. AgNPs was effectively able to remove the mature biofilms built up by P. aeruginosa. Moreover, AgNPs didn’t cause bacterial resistance, which is presumably due to that AgNPs do not exert their antibacterial effects only in a particular site but at several degrees such as bacterial wall, protein synthesis and DNA (Shrivastava et al., 2007). Silver has the highest levels of toxicity for microorganisms and the lowest toxicity for animal cells and AgNPs slowly release Ag+ ions that enables a constant local supply of Ag+ ions and allows an improved contact with the microorganisms. As a result, prevention of microorganisms’ adhesion and biofilm formation is more prolonged than in other antimicrobial approaches (Chen and Schluesener, 2008). Additionally, AgNPs causes oxidative damage, leading to the production of reactive oxygen species (ROS), i.e. free-radicals that is one of the primary mechanisms of toxicity to bacterial cells (Khan, 2012).This inhibitory effect of AgNPs on the built-up biofilm may due to be presence of water channels (pores) though out the biofilm, those are present for nutrient transportation and AgNPs may directly diffuse through the exopolysaccharide layer across these pores and reveal antimicrobial function (Kalishwaralal et al., 2010). The obtained results directly revealed that AgNPs (25µg/ml) not only effectively inhibited the growth of P. aeruginosa, but also wiped out the built-up biofilm. Therefore, the current experiment shows that AgNPs inhibited the formation of biofilm by P. aeruginosa alongside eliminate the biofilm formed previously. NaOCL is the most widely used oxidizing chemical for water disinfection and its mechanism of action is the removal of electrons from susceptible functional groups causing damage to bacterial cell, also has low molecular weight that able it to either pass through the membrane of bacterial cell and damage internal targets causing cell death or can disrupt the cell wall and membrane and cause bacterial death by that route (Finnegan et al., 2010). Therefore, the targets of the biocides can be the cell surface, cell wall or intracellular modules giving these agents a very broad spectrum of activity. NaOCL exhibited antibacterial activity against 4 P. aeruginosa strains isolated from poultry environment with MIC and MBC at 2200 µg/ml and 2600 µg/ml, respectively. Eriksson et al. (2017) revealed that NaOCL at concentrations of 0.01–0.08%, showed antibacterial effects against planktonic cells. The higher MIC value observed in the current study might be explained by differences in the origin of the bacterial isolates, as well as differences in the method of analysis. DeQueiroz and Day (2007) found that combination of NaOCL and hydrogen peroxide reduced P. aeruginosa ATCC 19142 cells numbers by 5-log to 6-log after 1 min exposure, by 7-log after 5 min exposure and no viable cells were detected after 20 min exposure. Heling et al. (2001) MIC and MBC of NaOCL against Streptococcus sobrinus, Streptococcus salivarius, Enterococcus faecalis and Streptococcus mutans were in the range of 0.157% to 0.315%. The obtained data revealed that NaOCL (from 2200 to2800 µg/ml) was able to promote a significant reduction in the biofilm building formed by all tested strains adhered to polystyrene wells and its efficiency was concentration dependent. This result was in line with the results reported by Russell and McDonnell (2000), who reported that the high concentrations of disinfectants were able to reduce more viable cells from biofilms, or even demonstrate 100% reduction in viable cells. However, the recorded result was much higher than that reported by Corcoran et al. (2014) who found that concentrations 250 mg/liter NaOCL was sufficient to inhibit growth of planktonic cells for studied salmonella strains. NaOCL was active in reducing the biofilm mass, making it a very valuable anti biofilm agent. Tote et al. (2010) reported that NaOCL was active on both biofilm matrix and viable mass of P. aeruginosa biofilms making them the superior antibiofilm agents. Additionally, the authors found that the activity was proportional to contact time, for which the anti-biofilms activity increased to 55% within the time 60 min., however, no complete reduction of the adherent populations was achieved even after 60 min of contact. As reported by Eginton et al. (1998) the attachment of the bacterial cells to surfaces was loosened by treatment with NaOCL. The obtained result showed that NaOCL (2800 µg/ml) exhibited 90.3%removalof the built-up biofilm on polystyrene wells after 1.5h of exposure. DeQueiroz and Day (2007) reported that treating pseudomonas biofilms on aluminum or stainless-steel plates with NaOCL and hydrogen peroxide mixture solution showed either a significant reduction or complete removal of biofilm material after a 5 min exposure. Buckingham- Meyer et al. (2007) found that NaOCL at 100, 500 and 1000mg/L resulted in a ~1-2 log10 reduction of Pseudomonas spp. biofilm (48 h) on glass surfaces. NaOCL at 100 and 200 mg/l was effective in killing or removing P. fluorescens adhered to stainless steel coupons after treatment for 10 min and the number of adhered cells was reduced from 52.0 to 0.0 (Rossoni and Gaylarde, 2000). NaOCL is the most commonly used disinfectant in the poultry farms and it is one of the most effective disinfectants against biofilms with the ability to eradicate biofilms at concentrations as low as 3.125 mg per ml (Pui et al., 2011; Rodrigues et al. 2011). Increasing NaOCl concentration and contact time increased the effectiveness of NaOCL against biofilms (Russell and McDonnell, 2000). Results from this study showed that NaOCl effectively eradicated the mature biofilms formed by P. aeruginosa. Although mature biofilm provides bacteria with special environment which is a protective factor against destructive agents. Therefore, the resistance of biofilms to antimicrobial agents increases with its aging (Diogo et al., 2015; Palaniswamy et al., 2016). The resistance is related to some factors as limited diffusion into biofilm structure, non-specific interaction with biofilm matrix and biofilm phenotype (Bridier et al., 2015). Finally, the current results revealed that AgNPs and NaOCl were able to promote a significant reduction on the biofilm formation adhered to polystyrene wells. Additionally, AgNPs and NaOCL effectively removed the mature biofilms formed by P. aeruginosa. and oxidizing agents as NaOCL is often used for the removal of biofilms (Meyer, 2003). As described by Russell (2007) NaOCL has disadvantages; activity greatly affected by pH (optimum is below 6.5), an irritating agent, inactivated by organic matter, carcinogenic byproducts with high corrosivity. In this context, there is a strong desire to develop other new strategies to control biofilm formation and according to Romero and Kolter (2014), even after many efforts reserved to develop new anti-adhesion agents, improve existing products and standard protocols are encouraged. Nanotechnology has emerged as an alternative tool for developing products with new properties to meet the increasing demand of the industrial sectors for advanced functional materials. Techniques as physical scrubbing of surfaces and the use of high-pressure water sprays are often applied for the removal of bacterial biofilms (Gibson et al., 1999). A problem is the generation of aerosols which results in the dispersion of the surviving micro-organisms (Holah et al., 1993). So, the chemical method to control biofilm is a popular approach (Simões et al., 2010). However, some chemical compounds as formaldehyde, peracetic acid, and mercuric chloride have been found to have no effect on biofilms (Carpentier and Cerf, 1993). In this respect AgNPs due to its cost effectiveness and high efficiency can be used to control biofilm. The current study emphasizes that the correct and conscious of cleanliness, hygiene, sanitation, and health education of workers must be daily and continuous, in addition to equipment maintenance and periodic exchange and treatment of equipment. If these parameters are checked consistently, the installation process of the biofilm is hindered and, thus, the persistence of pathogenic bacteria with the constant contamination of the poultry and their environment will be reduced and may even be eradicated. |
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
Conclusion |
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
The observations of the current study are significant as AgNPs were shown to be effective against biofilms of P. aeruginosa isolated from chicken farms. Thus, AgNPs might be a good alternative antibacterial agent against the multidrug resistant bacteria and the applications of AgNPs may lead to valuable findings in various fields such as medical devices and antimicrobial systems. |
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
Conflict of Interests |
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
Authors do not have conflicts of interest to disclose. |
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
References |
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
Abidi, S.H., Ahmed, K., Sherwani, S.K., Kazmi, S.U., 2014. Reduction and removal of Pseudomonas aeruginosa biofilm by natural agents. Int. J. Chem. Pharm. Sci, 5, 28-34. Alionte, L.G., Cannon, B.M., White, C.D., Caballero, A.R., O'Callaghan, R.J., Hobden, l.A., 2001. Pseudomonas aeruginosa LasA protease and corneal infections. Current Eye Research 22, 266-271 Amirulhusni, A.N., Palanisamy, N.K., Mohd-Zain, Z., Ping, L.J., Durairaj, R., 2012. Antibacterial Effect of Silver Nanoparticles on Multi-Drug Resistant Pseudomonas Aeruginosa. International Journal of Medical, Health, Biomedical, Bioengineering and Pharmaceutical Engineering 6, 291-294. Anderl, J.N., Zahller, J., Roe, F., Stewart, P.S., 2003. Role of nutrient limitation and stationary-phase existence in Klebsiella pneumoniae biofilm resistance to ampicillin and ciprofloxacin. Antimicrobial Agents and Chemotherapy 47, 1251-1256.þ Bentzmann, S, Plésiat, P., 2011. The Pseudomonas aeruginosa opportunistic pathogen and human infections. Environ Microbiol 13, 1655-1665. Bridier, A., Sanchez-Vizuete, P., Guilbaud, M., Piard, J.C., Naïtali, M., Briandet, R., 2015. Biofilm-associated Persistence of Food-borne Pathogens. Food Microbiology 45, 167-178. Buckingham-Meyer, K., Goeres, D.M., Hamilton, M.A., 2007. Comparative evaluation of biofilm disinfectant efficacy tests. Journal of Microbiological Methods 70, 236-244.þ Carpentier, B., Cerf, O., 1993. Biofilms and their consequences, with particular reference to hygiene in the food industry. J. Appl. Bacteriol. 75, 499–511. Chen, X., Schluesener, H.J., 2008. Nanosilver: a nanoproduct in medical application. Toxicology Letters 176, 1-12.þ Cherif-Antar, A., Moussa–Boudjemâa, B., Didouh, N., Medjahdi, K., Mayo, B., Flórez, A.B., 2016. Diversity and biofilm-forming capability of bacteria recovered from stainless steel pipes of a milk-processing dairy plant. Dairy Science and Technology 96, 27-38.þ Coffey, B.M., Anderson, G.G., 2014. Biofilm formation in the 96-well microtiter plate. Pseudomonas Methods and Protocols 1149, 631-641. Corcoran, M., Morris, D., De Lappe, N., O’Connor, J., Lalor, P., Dockery, P., Cormican, M., 2014. Commonly used disinfectants fail to eradicate Salmonella enterica biofilms from food contact surface materials. Appl. Environ. Microbiol. 80, 1507–1514. Corry, J.E., Curtis, G.D., Baird, R.M., 2011. Handbook of culture media for food and water microbiology. Royal Society of Chemistry. þ Cambridge, United Kingdom. de Faria, A.F., Martinez, D.S.T., Meira, S. M.M., de Moraes, A.C.M., Brandelli, A., Filho, A. G.S., Alves, O.L., 2014. Anti-adhesion and antibacterial activity of silver nanoparticles supported on graphene oxide sheets. Colloids and Surfaces Biointerfaces 113,115-124 DeQueiroz, G.A. Day, D.F., 2007. Antimicrobial activity and effectiveness of a combination of sodium hypochlorite and hydrogen peroxide in killing and removing Pseudomonas aeruginosa biofilms from surfaces Journal of Applied Microbiology 103, 794–802 Diogo, P., Gonçalves, T., Palma, P., Santos, J.M., 2015. Photodynamic antimicrobial chemotherapy for root canal system asepsis: A narrative literature review. International Journal of Dentistry :269205. (http://dx.doi.org/10.1155/2015/269205) Eginton, P.J., Holah, J., Allison, D.G., Handley, P.S., Gilbert, P., 1998. Changes in the strength of attachment of microorganisms to surfaces following treatment with disinfectants and cleansing agents. Lett. Appl. Microbiol 27, 101–105. Elliott, T.S.J., 2007. An update on antimicrobial central venous catheters. Journal of Hospital Infection 65, 34-38 Eriksson, L., Holgerson, P.L., Johansson, I., 2017. Saliva and tooth biofilm bacterial microbiota in adolescents in a low caries community. Scientific Reports 7, 5861.
Fabrega, J., Zhang, R., Renshaw, J.C., Liu, W.T., Lead, J.R., 2011. Impact of silver nanoparticles on natural marine biofilm bacteria. Chemosphere 85, 961-966. Finnegan, M., Linley, E., Denyer, S.P., McDonnell, G, Simons, C., Maillard, J.Y., 2010. Mode of action of hydrogen peroxide and other oxidizing agents: differences between liquid and gas forms. Journal of Antimicrobial Chemotherapy 65, 2108–2115. GanjaliDashti, M., Abdeshahian, P., Sudesh, K., Phua, K.K., 2016. Optimization of Salmonella Typhi biofilm assay on polypropylene microtiter plates using response surface methodology. Biofouling 32, 477-487. Gibson, H., Taylor, J., Hall, K., Holah, J., 1999. Effectiveness of cleaning techniques used in the food industry in terms of the removal of bacterial biofilms. Journal of Applied Microbiology 87, 41–48. Habash, M.B., Park, A.J., Vis, E.C., Harris, R.J., Khursigara, C.M., 2014. Synergy of silver nanoparticles and aztreonam against Pseudomonas aeruginosa PAO1 biofilms. Antimicrobial agents and chemotherapy 58, 5818-5830.þ Hall‐Stoodley, L., Stoodley, P., 2009. Evolving concepts in biofilm infections. Cellular Microbiology 11, 1034-1043. Hawkins, C.L., Davies, M.J., 1999 Hypochlorite-induced oxidation of proteins in plasma: formation of chloramines and nitrogen-centred radicals and their role in protein fragmentation. Biochemical Journal 340, 539-548.þ Heling, I., Rotstein, I., Dinur, T., Szwec-Levine, Y., 2001. Bactericidal and cytotoxic effects of sodium hypochlorite and sodium dichloroisocyanurate solutions in vitro. Journal of Endodontics 27, 278-280.
Holah, J., Taylor, J., Holder, J., 1993. The spread of Listeria by cleaning systems. Campden& Chorleywood Food Research Association. Technical memorandum No. 673. Ito, A., Taniuchi, A., May, T., Kawata, K., Okabe, S., 2009. Increased antibiotic resistance of Escherichia coli in mature biofilms. Applied and Environmental Microbiology 75, 4093-4100.þ Kalishwaralal, K., BarathManiKanth, S., Pandian, S.R.K., Deepak, V., Gurunathan, S., 2010. Silver nanoparticles impede the biofilm formation by Pseudomonas aeruginosa and Staphylococcus epidermidis. Colloids and Surfaces B: Biointerfaces 79, 340-344.þ Kebede, F., 2010. Pseudomonas infection in chickens. Journal of Veterinary Medicine and Animal Health 2, 55-58.þ Khan, A.U., 2012. Medicine at nanoscale: a new horizon. International journal of nanomedicine 7, 2997.þ Kora, A.J., Arunachalam, J., 2011. Assessment of antibacterial activity of silver nanoparticles on Pseudomonas aeruginosa and its mechanism of action World. J. Microbiol. Biotechnol. 27, 1209–1216 Kumar, R., Chhibber, S., Harjai, K., 2009. A Comparative Study of Clinical and Environmental Isolates of Pseudomonas aeruginosa in Terms of Quorum Sensing, Outer Membrane Proteins and Their Ability to Cause Urinary Tract Infection 1, 205–214. Lavenir, R., Jocktane, D., Laurent, F., Nazaret, S., Cournoyer, B., 2007. Improved reliability of Pseudomonas aeruginosa PCR detection by the use of the species-specific ecfX gene target. Journal of Microbiological Methods 70, 20–29. Marchand, S., De Block, J., De Jonghe, V., Coorevits, A., Heyndrickx, M., Herman, L., 2012. Biofilm formation in milk production and processing environments; influence on milk quality and safety. Compr. Rev. Food Sci. Food Saf. 11 133–147. Martinez-Gutierrez, F., Boegli, L., Agostinho, A., Sánchez, E.M., Bach, H., Ruiz, F., James, G., 2013. Anti-biofilm activity of silver nanoparticles against different microorganisms. Biofouling 29, 651-660.þ McLaughlin-Borlace, L., Stapleton, F., Matheson, M., Dart, J.K.G., 1998. Bacterial biofilm on contact lenses and lens storage cases in wearers with microbial keratitis. Journal of Applied Microbiology 84, 827-838. Melchior, M.B., Fink‐Gremmels, J., Gaastra, W., 2006. Comparative assessment of the antimicrobial susceptibility of Staphylococcus aureus isolates from bovine mastitis in biofilm versus planktonic culture. Zoonoses and Public Health 53, 326-332.þ Mena, K.D., Gerba, C.P., 2009. Risk Assessment of Pseudomonas aeruginosa in water. Rev Environ. Contam. Toxicol. 201,71-115. Meyer, B., 2003. Approaches to prevention, removal and killing of biofilms. Int. Biodeter. Biodegr 51, 249–253. Mohanty, S., Mishra, S., Jena, P., Jacob, B., Sarkar, B., Sonawane, A., 2012. An investigation on the antibacterial, cytotoxic, and antibiofilm efficacy of starch-stabilized silver nanoparticles. Nanomedicine: Nanotechnology, Biology and Medicine 8, 916-924.þ Monds, R.D., O’Toole, G.A., 2009. The developmental model of microbial biofilms: ten years of a paradigm up for review. Trends in Microbiology 17, 73-87.þ Morones, J.R., Elechiguerra, J.L., Camacho, A., Holt, K., Kouri, J.B., Ramírez, J.T., Yacaman, M.J., 2005. The bactericidal effect of silver nanoparticles. Nanotechnology 16, 2346-2353.þ O'Toole, G.A. Kolter, R., 1998. Flagellar and twitching motility are necessary for Pseudomonas aeruginosa biofilm development. Mol. Microbiol. 30, 295-304. O’Toole, G., Kaplan, H.B., Kolter, R., 2000. Biofilm formation as microbial development. Annu. Rev. Microbiol. 54, 49–79. Otter, J.A., Vickery, K., Walker, J.D., deLancey Pulcini, E., Stoodley, P., Goldenberg, S.D., Edgeworth, J.D., 2015. Surface-attached cells, biofilms and biocide susceptibility: implications for hospital cleaning and disinfection. Journal of Hospital Infection 89, 16-27. Pal, S., Tak, Y.K., Song, J.M., 2007. Does the antibacterial activity of silver nanoparticles depend on the shape of the nanoparticle? A study of the gram-negative bacterium Escherichia coli. Applied and Environmental Microbiology 73, 1712-1720.þ Palanisamy, N.K., Ferina, N., Amirulhusni, A.N., Mohd-Zain, Z., Hussaini, J., PingL, J., Durairaj, R., 2014. Antibiofilm properties of chemically synthesized silver nanoparticles found against Pseudomonas aeruginosa. Journal of Nanobiotechnology 12:2 Palaniswamy, U., Lakkam, S.R., Arya, S., Aravelli, S., 2016. Effectiveness of N-acetyl cysteine, 2% chlorhexidine, and their combination as intracanal medicaments on Enterococcus faecalis biofilm. Journal of Conservative Dentistry 19, 17.þ Peix, A., Ramírez-Bahena, M.H., Velázquez, E., 2009. Historical evolution and current status of the taxonomy of genus Pseudomonas. Infection, Genetics and Evolution 9, 1132-1147.þ Pollack, M., 2000. Pseudomonas aeruginosa. In: Principles and Practice of Infectious Diseases (Mandell, G.L., Benett, I.E., Dolin R., eds). Churchill Livingstone, New York. pp. 2310-2335. Pui, C. F., Wong, W. C., Chai, L. C., Tunung, R., Jeyaletchumi, P., Hidayah, N., Son, R., 2011. Salmonella: A foodborne pathogen. International Food Research Journal 18, 465-473 Radzig, M.A., Nadtochenko, V.A., Koksharova, O.A., Kiwi, J., Lipasova, V.A., Khmel, I.A., 2013. Antibacterial effects of silver nanoparticles on gram-negative bacteria: influence on the growth and biofilms formation, mechanisms of action. Colloids and Surfaces B: Biointerfaces, 102, 300-306.þ Rai, M., Yadav, A., Gade, A., 2009. Silver nanoparticles as a new generation of antimicrobials. Biotechnology Advances 27, 76-83.þ Rocchetta, H.L., Burrows, L.L., Lam, I.S., 1999. Genetics of O-antigen biosynthesis in Pseudomonas aeruginosa. Microbiol. Mol. Bioi. Rev. 63, 523–553 Rodrigues, D., Cerca, N., Teixeira, P., Oliveira, R., Ceri, H., Azeredo, J., 2011. Listeria monocytogenes and Salmonella enterica Enteritidis biofilms susceptibility to different disinfectants and stress-response and virulence gene expression of surviving cells. Microbial Drug Resistance 17, 181-189 Romero, D. Kolter, R., 2014. Functional amyloids in bacteria.International Microbiology. 17:65-73 Rossoni, E.M.M., Gaylarde, C.C., 2000. Comparison of sodium hypochlorite and peracetic acid as sanitizing agents for stainless steel food processing surfaces using ifluorescence microscopy. International Journal of Food Microbiology 61, 81–85. Roy, R., Tiwari, M., Donelli, G., Tiwari, V., 2017. Strategies for combating bacterial biofilms: A focus on anti-biofilm agents and their mechanisms of action. Virulence 9, 522–554 Russell S.M., 2007. Chlorine is still the most popular sanitizer in the poultry industry (part 1). Cooperative Extension Service, College of Agricultural and Environmental Sciences / Athens, Georgia 30602-4356. Russell, A.D., McDonnell, G., 2000. Concentration: a major factor in studying biocidal action. Journal of Hospital Infection 44, 1-3.þ Saldaña, Z., Xicohtencatl‐Cortes, J., Avelino, F., Phillips, A.D., Kaper, J.B., Puente, J. L., Girón, J.A., 2009. Synergistic role of curli and cellulose in cell adherence and biofilm formation of attaching and effacing Escherichia coli and identification of Fis as a negative regulator of curli. Environmental Microbiology 11, 992-1006.þ Secinti, K.D., Özalp, H., Attar, A., Sargon, M.F., 2011. Nanoparticle silver ion coatings inhibit biofilm formation on titanium implants. Journal of Clinical Neuroscience 18, 391–395 Sharma, B. K., Saha, A., Rahaman, L., Bhattacharjee, S., Tribedi, P., 2015. Silver inhibits the biofilm formation of Pseudomonas aeruginosa. Advances in Microbiology, 5, 677-685.þ Shrivastava, S., Bera, T., Roy, A., Singh, G., Ramachandrarao, P., Dash, D., 2007. Characterization of enhanced antibacterial effects of novel silver nanoparticles. Nanotechnology 18, 225103-225112. Shukla, S., Mishra, P., 2015. Pseudomonas aeruginosa Infection in Broiler Chicks in Jabalpur International J. Ext. Res. 6, 37-39. Simões, M., Simões, L. C., Vieira, M., 2010. A review of current and emergent biofilm control strategies. LWT-Food Science and Technology 43, 573-583. Singh, K., Panghal, M., Kadyan, S., Chaudhary, U., Yadav, J.P., 2014. Antibacterial Activity of Synthesized Silver Nanoparticles from Tinosporacordifolia against Multi Drug Resistant Strains of Pseudomonas aeruginosa Isolated from Burn Patients. J. Nanomed Nanotechnol, 5, 192. Stellato, G., De Filippis, F., La Storia, A., Ercolini, D., 2015. Coexistence of lactic acid bacteria and potential spoilage microbiota in a dairy processing environment. Appl. Environ. Microbiol. 81, 7893–7904. Suresh, T., Hatha, A.A.M., Harsha, H.T., Lakshmanaperumalsamy, P., 2011. Prevalence and distribution of Salmonella serotypes in marketed broiler chickens and processing environment in Coimbatore City of southern India. Food Research International 44, 823-825.þ Thuptimdang, P., Limpiyakorn, T., McEvoy, J., Prüß, B.M., Khan, E., 2015. Effect of silver nanoparticles on Pseudomonas putida biofilms at different stages of maturity. J. Hazard. Mater. 290, 127–133. Tote, K., Horemans, T., Berghe, D.V., Maes, L., Cos, P., 2010. Inhibitory effect of biocides on the viable masses and matrices of Staphylococcus aureus and Pseudomonas aeruginosa biofilms. Applied and Environmental Microbiology 76, 3135-3142. Versalovic J., Carroll K.X., Gunde G., Jorgensen J.H., Landry M.L., Warnock D.W., 2011. Manual of clinical microbiology, 10th ed. American Society for Microbiology, Washington, DC. Vigneshwaran, N., Nachane, R.P., Balasubramanya, R.H., Varadarajan, P.V., 2006. A novel one-pot ‘green’ synthesis of stable silver nanoparticles using soluble starch. Carbohydrate Research 341, 2012-2018.þ Wagner, V.E., Iglewski, B.H., 2008. P. aeruginosa biofilms in CF infection. Clinical Reviews in Allergy and Immunology 35, 124-134.þ Winn, W.C., 2006. Koneman's color atlas and textbook of diagnostic microbiology. 6th ed. Lippincott Williams and Wilkins. Wong, H.S., Townsend, K.M., Fenwick, S.G., Trengove, R.D., O’handley, R.M., 2010. Comparative susceptibility of planktonic and 3‐day‐old Salmonella Typhimurium biofilms to disinfectants. Journal of Applied Microbiology 108, 2222-2228.þ Yarwood, J.M., Schlievert, P.M., 2003. Quorum sensing in Staphylococcus infections. Journal of Clinical Investigation 112, 1620-1625. |
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||