|
|
Journal of Advanced Veterinary Research Volume 10, Issue 1, 2020, Pages: 1-8 www.advetresearch.com |
|
|
Prevalence, Distribution Pattern and Pathological Alterations of Gastrointestinal Helminthosis in Domestic Ducks in Beni-Suef, Egypt |
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
Khaled Mohamed El-Dakhly1*, Hend Ibrahim Mohamed1, Asmaa Alaa Kamel1, Lilian N. Mahrous1, El-Shaymaa El-Nahass2, Amany Samir Mohamed Aboshinaf3 |
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
1Department of Parasitology, Faculty of Veterinary Medicine, Beni-Suef University, Beni-Suef 62511, Egypt 2Department of Pathology, Faculty of Veterinary Medicine, Beni-Suef University, Beni-Suef 62511, Egypt 3Provincial Laboratory of Animal Health Research Institute, Dokki, Giza, Egypt. |
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
Received: 3 December 2019; Accepted 28 December 2019. |
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
(*: Corresponding author: eldakley_s71@yahoo.com) |
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
Abstract |
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
` In Egypt, scarce literature on helminthosis in aquatic birds, particularly ducks, were reported. Therefore, the current study was conducted to explore the prevalence of helminth infections, and the associated histopathological alterations, in domestic ducks in Beni-Suef, Egypt. Accordingly, a total of 510 ducks (260 native, 150 mallard and 100 Muscovy) were collected from villages and local markets to screen the gastrointestinal helminthosis during the period from October 2018 to November 2019. It was found that the overall prevalence was 13.92% (71/510). Among those, 11 (2.16%) ducks had mixed infections. The recovered species were 8 tapeworm species and 5 nematode species. Among tapeworms, Raillietina tetragona was the most prevalent (1.96%; 10/510) followed by R. cesticillus (1.57%; 8/510), Amoebotaenia cuneata (1.18%;6/510), Cotugnia digonopora (0.98%;5/510), R. echinobothrida (0.78%; 4/510), Hymenolepis apodemi-like (0.78%; 4/510), Choanotaenia infundibulum (0.59%; 3/510) and H. carioca (0.39%; 2/510). Among nematodes, the most prevalent species was Ascaridia galli (5.10%; 26/510) followed by Heterakis gallinarum (1.76%; 9/510), Subulura brumpti (0.59%; 3/510), Trichostrongylus tenuis (0.2%; 1/510) and Epomidiostomum uncinatum (0.2%; 1/510). The highest prevalence was recorded in native breed, while the lowest was in Muscovy ducks. Seasonally, the highest prevalence was detected in autumn and summer, while the lowest infection rate was recorded in winter. Histopathologically, diffuse degenerative changes and necrosis of intestinal mucosa as well as hyalinosis of the muscular layer were predominant. Further studies on other aquatic birds in Egypt are urgently demanded to verify helminth parasites posing on the associated risk factors to minimize economic losses resulted from mortalities induced by those parasitic infections. Moreover, regular control programming including effective treatment is highly recommended. |
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
Keywords: Helminths, Ducks, Egypt, Prevalence, Seasonal dynamics |
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
Introduction |
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Two breeds of ducks are reared worldwide; the Muscovy ducks (Cairina moschata) and the mallard ducks (Anas platyrhynchus) (Harrison and Greensmith, 1993). In Egypt, various breeds of ducks are present; native, Sudanese, and white Peckin, however, Muscovy and Campbell are newly introduced breeds. Domestic ducks tend to be more contact with humans and birds as a source of protein (Cooper, 1984; Harlin, 1994; Radfar et al., 2011).
Ducks are susceptible to infection with large number of intestinal helminths. They act as the final and intermediate hosts for various protozoon and helminth parasites (Gicik and Arslan, 2003; Olsen, 2009). Such parasites have serious effects on the health and result in economic losses in the form of a decrease in body weight, reduction in egg production and increased the susceptibility to infectious diseases (AbouLaila et al., 2011). Stunted growth, emaciation and death are the common symptoms in young birds. Mature ducks may appear symptomless during the course of infection (Gicik and Arslan, 2003; Wang et al., 2004). However, parasitic infections in ducks are frequently ignored in breeding management. Common helminths of ducks include nematodes (roundworms), trematodes (flukes), and cestodes (tapeworms). The nematodal infection is through direct ingestion of eggs or larvae passed from feces of the final host (Cole and Friend, 1999). Oppositely, tapeworms and digenean trematodes need at least one intermediate host to complete the life cycle. Intermediate host ingest eggs and larvae expelled from the final host to develop into an infective stage. Then, final hosts ingest intermediate hosts to complete the cycle (Cole and Friend, 1999). Several factors affecting helminth transmission and permanence include rainfall, humidity, soil and water temperature as well as accessibility of intermediate and final hosts (Poulin, 2006; Dudley et al., 2015). So, due to being little is known about parasitic infections, particularly helminths, in ducks in Egypt, the objective of such study was to determine the prevalence and distribution pattern of intestinal helminths as well as their induced pathological alterations in domestic ducks in Beni-Suef province, Egypt. The helminth species recovered were identified and effect of the seasonal dynamics was investigated too. |
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
Materials and methods |
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
Study area and sampling In the current investigation, intestinal tracts from 510 necropsied domestic ducks of different breads (native, mallard and Muscovy) were collected from different local markets to investigate intestinal helminthosis in Beni-Suef province (coordinates: 29°04′N 31°05′E), Egypt during the period from October 2018 to November 2019. Samples preparation and necropsy Collected samples were transported to the laboratory of Parasitology, Faculty of Veterinary Medicine, Beni-Suef University for necropsy. The intestinal tract was divided into foregut, midgut and hindgut. Each part was opened along the line of the lesser curvature and examined separately with its contents in a large clean Petri dish containing normal saline. The macroscopic worms were collected and transferred into another Petri dish containing normal saline. The mucosa of each part was scraped, and then examined under the dissecting microscope. The remnants of the intestinal contents was transferred into a cylinder containing physiological saline and left for 30-60 minutes to permit the content to settle down. The supernatant fluid was poured off leaving the sediment, which was examined. The sediment was poured into a small Petri dish and examined under the dissecting microscope. The collected worms were left in the refrigerator for 4-12 hours for a complete relaxation (El-Dakhly et al., 2012; Ahmed et al., 2013). Parasitological examinations Tapeworms were dorsoventrally compressed between two glass slides with gentle pressure, and then fixed in neutral buffered formalin 10%. The time of fixation varied from 4 hours for small samples to 24 hours for large ones. The fixed worms were washed several times by tap water and stained by acetic acid alum carmine followed by dehydration in ascending grades of ethyl alcohol, then cleared in xylene and mounted in Canada balsam on clean glass slides with cover slips (El-Dakhly et al., 2012; Ahmed et al., 2013; Rzad et al., 2013). Roundworms were preserved in 70% ethyl alcohol containing 5% glycerin (Ahmed et al., 2013) then cleared in lactophenol and mounted in glycerol jelly (El-Dakhly et al., 2012). Prepared slides were carefully examined under a light microscopy and recovered helminths were identified according to Yamaguti (1961) and Soulsby (1982). The overall prevalence, average abundance and their distribution of collected helminths in the intestinal tract were evaluated (Margolis et al., 1982). The mean intensity= Total number of particular helminth species in a duck/Number of infected ducks. The prevalence of helminth infection= Number of ducks infected with a particular helminth species/ Number of ducks examined. Histopathology After removal of both tapeworms and nematodes, the mucosal surface of the infected intestinal tract was taken for histopathological examination. Pieces of the intestine were fixed in 10% neutral buffered formalin, processed routinely for paraffin embedding, sectioned at 5 mm and stained with haematoxylin and eosin (HE) for photomicroscopy (Bancroft and Stevens, 1996; El-Dakhly et al., 2012). Statistical analysis The prevalence and mean intensity were applied as described by Margolis et al. (1982). Chi-square test (χ2) was employed to determine the possible association of parasite prevalence relative to breeds and seasons. |
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
Results |
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
The current investigation revealed that out of 510 examined ducks of different breeds (native, mallard and Muscovy), 71 (13.92%) were infected with gastrointestinal helminths. Ducks were infected by 13 species of helminths. The recovered helminths were recognized as tapeworms (42/510; 8.24%) and nematodes (40/510; 7.84%). No trematodes could be detected. Mixed infections were recorded in 2.16% of examined birds (Table 1). Furthermore, the prevalence of helminths among native ducks was 17.31%, and that among mallard ducks was 13.33% but the infection rate among Muscovy ducks was 6%. Tapeworms were found in prevalences of 11.92%%, 6% and 2% in native, mallard and Muscovy ducks, respectively, while nematodes were recovered in infection rates of 9.62, 7.33 and 4%, respectively (Table 2). Table 1. The overall prevalence of helminth infections in examined ducks in Beni-Suef, Egypt.
No.: Number of infected ducks, %: Percentage of infection
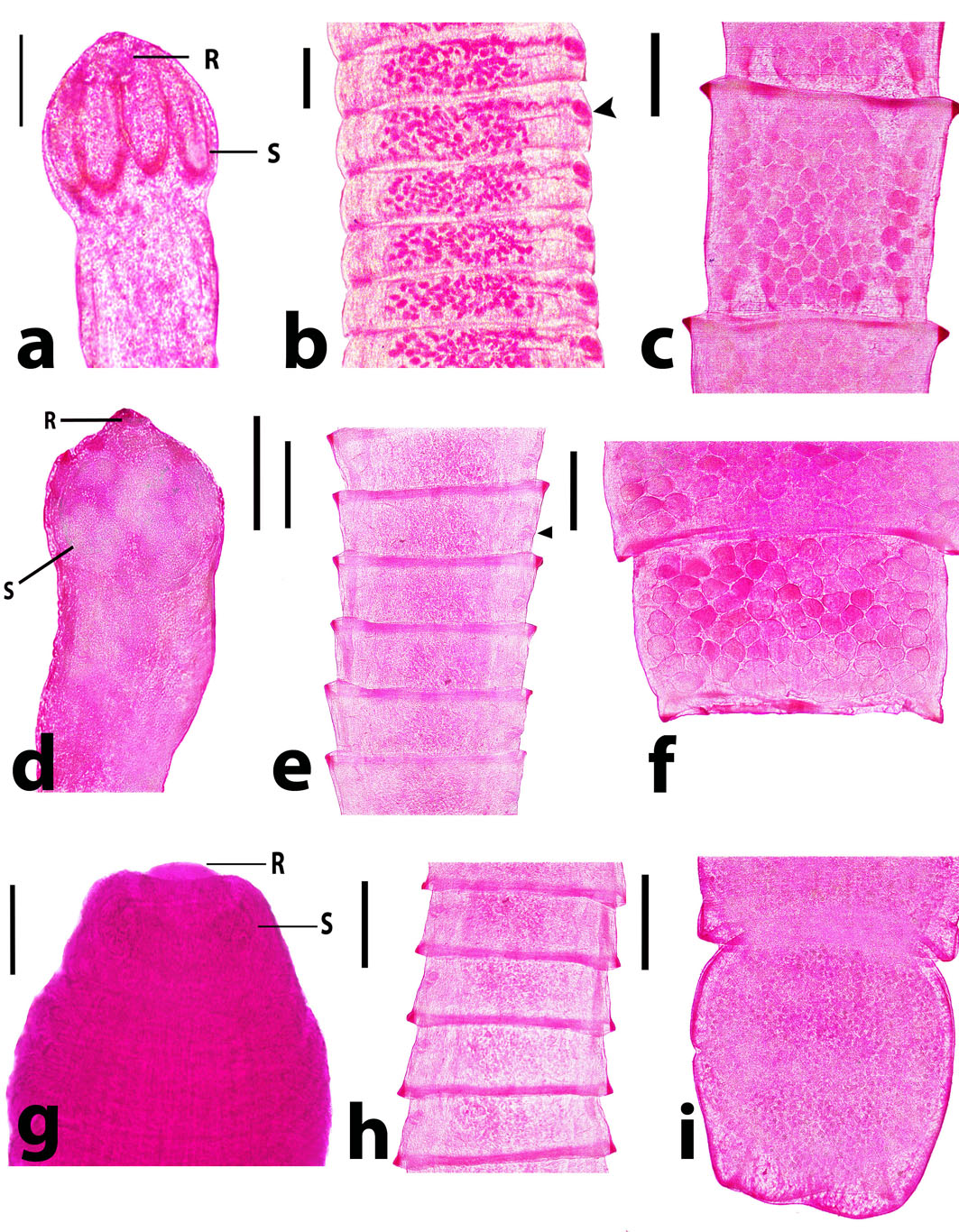
Fig. 1. Raillietina spp. recovered from domestic ducks. a) R. tetragona scolex showing 4 oval-shaped suckers (S) and rostellum (R) armed with minute hooks. Scale bar= 150 µm. b) R. tetragona mature proglottids with unilateral common genital pores anterior to the middle part (arrowhead). Scale bar= 500 µm. c) R. tetragona gravid proglottids with egg capsules containing several ova. Scale bar= 500 µm. d) R. echinobothrida scolex with circular-shaped suckers (S) and rostellum (R) heavily armed. Scale bar= 200 µm. e) R. echinobothrida mature proglottids with unilateral common genital pores posterior to the middle part (arrowhead). Scale bar= 500 µm. f) R. echinobothrida gravid proglottids with egg capsules containing several ova. Scale bar= 500 µm. g) R. cesticillus scolex with rounded unarmed suckers (S) and retractable and piston-like rostellum (R). Scale bar= 100 µm. h) R. cesticillus mature proglottid with genital pores anterior to the middle part. Scale bar= 500 µm. i) R. cesticillus gravid proglottid with egg capsules containing several ova. Scale bar= 500 µm. Table 2. The overall prevalence of intestinal helminths among different breeds of ducks
No.: Number of infected ducks; %: Percentage of infection; P value ˃ 0.05: non-significant (NS)
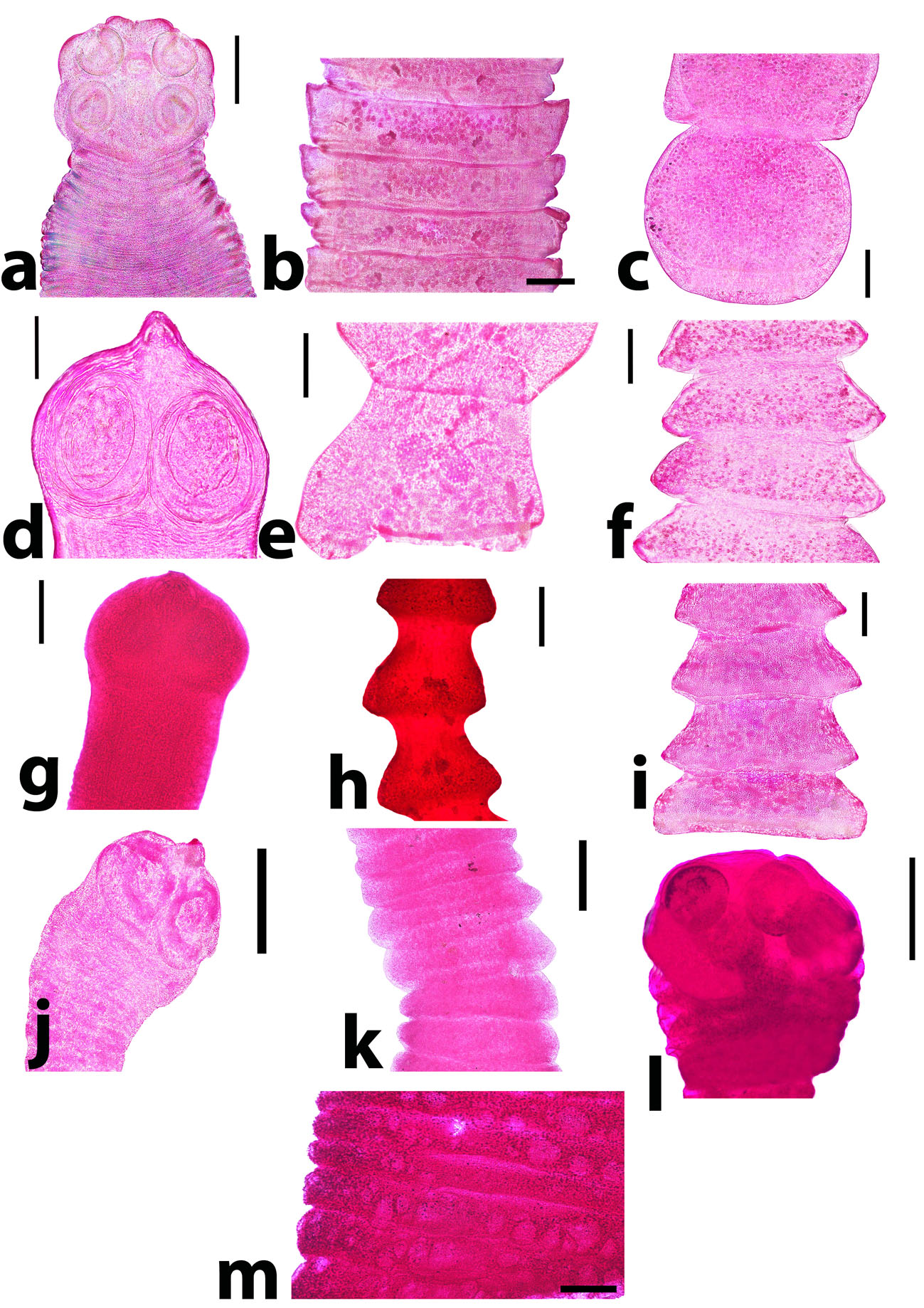
Fig. 2. Adult cestodes rather than Raillietina spp. revealed from ducks. a) Cotugnia digonopora scolex. Scale bar= 500 µm. b) C. digonopora mature proglottid. Scale bar= 500 µm. c) C. digonopora gravid proglottid. Scale bar= 500 µm. d) Amoebotaenia cuneata scolex. Scale bar= 100 µm. e) A. cuneata mature proglottid. Scale bar= 200 µm. f) A. cuneata gravid proglottid. Scale bar= 200 µm. g) Choanotaenia infundibulum scolex. Scale bar= 200 µm. h) Ch. infundibulum mature proglottid. Scale bar= 200 µm. i) Ch. infundibulum gravid proglottid. Scale bar= 200 µm. j) Hymenolepis carioca scolex. Scale bar= 200 µm. k) H. carioca strobila. Scale bar= 200 µm. l) Hymenolepis apodemi-like scolex. Scale bar= 200 µm. m) H. apodemi-like pregravid proglottid. Scale bar= 200 µm. Currently, 8 species of cestodes were identified. The most prevalent species was Raillietina tetragona (10/510; 1.96%) followed by Raillietina cesticillus (8/510; 1. 57%) and Amoebotaenia cuneata (6/510; 1.18%). The lower abundant tapeworms were Cotugnia digonopora (5/510; 0.98%), Raillietina echinobothrida (4/510; 0.78%), Choanotaenia infundibulum (3/510; 0.59%), Hymenolepis apodemi-like (4/510; 0.78%) and Hymenolepis carioca (2/510; 0.39%). It has been found that Amoebotaenia cuneata had the highest intensity among the recovered helminths, while Hymenolepis carioca showed the lowest one (Table 3 and Figs. 1, 2). Table 3. The distribution and intensity of helminth infections in ducks.
Concerning roundworms, five species were identified. The most prevalent species was Ascaridia galli (26/510; 5.10%) followed by Heterakis gallinarum (9/510; 1.76%). The least common nematodes were Subulura brumpti (3/510; 0.59%), Trichostrongylus tenuis (1/510; 0.20%) and Epomidiostomum uncinatum (1/510; 0.20%). It is worthy to mention that Heterakis gallinarum had the highest intensity among the recovered nematode helminths (Table 3 and Figs. 3, 4).
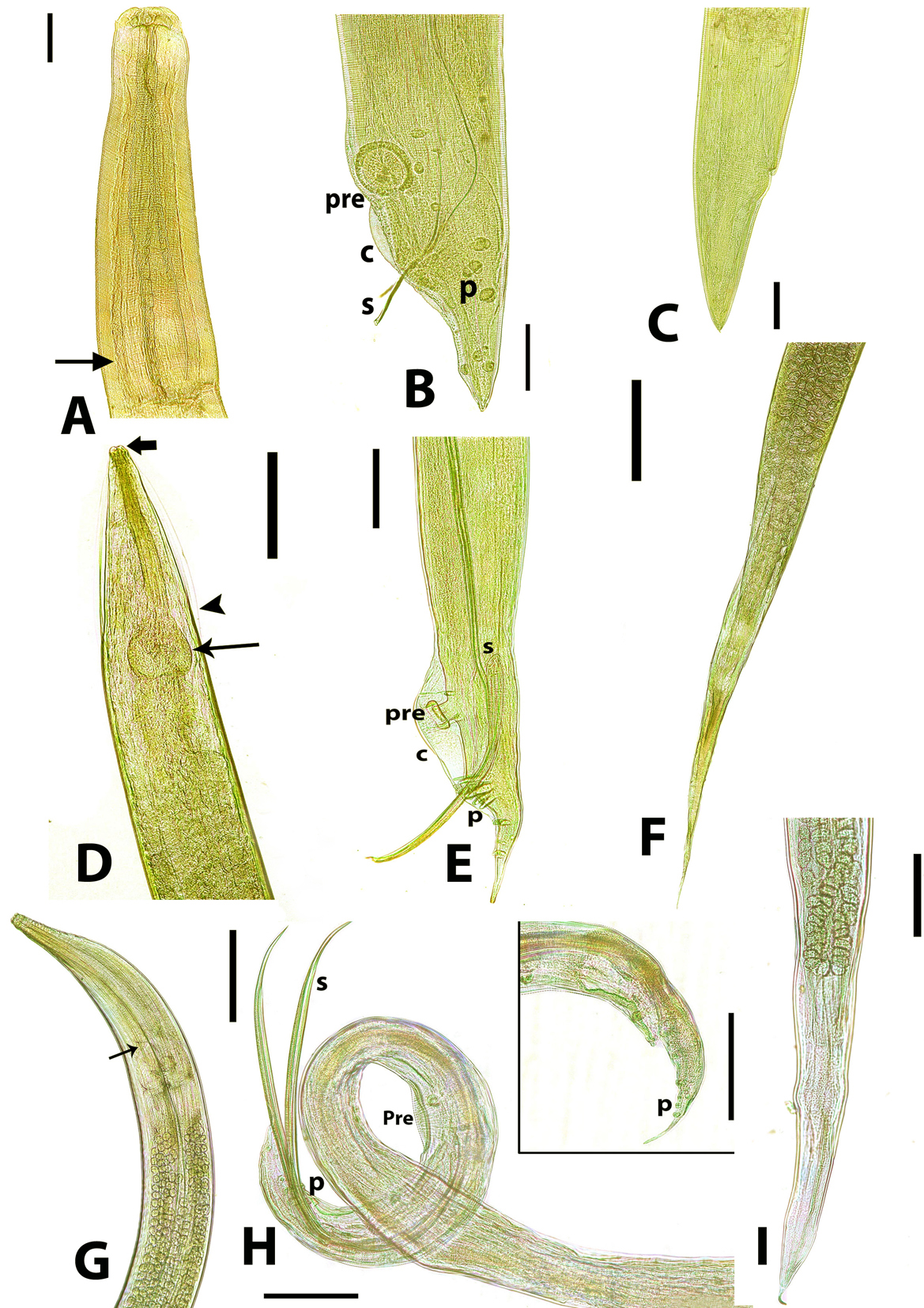
Fig. 3. Adult nematodes recovered from necropsied domestic ducks. A) Anterior end of adult Ascaridia galli showing a simple club-shaped oesophagus. Scale bar= 500 µm. B) A. galli adult male posterior end showing subequal spicules (s), slit-like precloacal sucker (pre), narrow caudal alae (c) and well-developed caudal papillae (p). Scale bar= 200 µm. C) A. galli adult female showing straight and conical posterior end. Scale bar= 500 µm. D) Anterior end of adult Heterakis gallinarum showing reduced lips (thick arrow), narrow lateral alae (arrowhead) and a strong posterior bulb-shaped oesophagus (arrow). scale bar= 500 µm. E) H. gallinarum adult male posterior end showing unequal spicules (s), prominent and circular precloacal sucker (pre), large and well-developed caudal alae (c) and caudal papillae (p). Scale bar= 200 µm. F) H. gallinarum adult female showing a pointed and tapered posterior end. Scale bar= 500 µm. G) Anterior end of adult Subulura brumpti. Arrow denotes a clear constriction indicating a double-bulb-shaped oesophagus. Scale bar= 500 µm. H) S. brumpti adult male posterior end showing equal spicules (s), elongate slit-shaped precloacal sucker (pre). Scale bar= 200 µm. Inset: caudal papillae (p). Scale bar= 200 µm. I) S. brumpti female posterior end showing less pointed posterior end. Scale bar= 500 µm. Seasonally, it has been found that the highest prevalence of duck helminthosis was found in autumn (19.18%; 14/73), while the infection rate declined in other seasons; 13.46% (28/208) in spring, 13.33% (12/90) in summer and 12.14%; 17/140) in winter (Table 4). Table 4. The seasonal prevalence of cestodes and nematodes among ducks in Beni-Suef province, Egypt.
No. = Number of infected ducks % = Percentage of infection P value ˃ 0.05 is non-significant (NS) *: The total infected birds 14 including 11 ducks with mixed infection and 3 individuals had tapeworms only Among cestodes, it was observed that Raillietina tetragona was more prevalent in autumn, however, Raillietina cesticillus was found in low percent in both spring and winter. Raillietina echinobothrida was found in spring and autumn only. Amoebotaenia cuneata was detected in a lower prevalence in summer, autumn and winter. Furthermore, Cotugnia digonopora and Hymenolepis apodemi-like were recovered only in summer and autumn. Choanotaenia infundibulum and Hymenolepis carioca were recorded in spring only. Concerning the nematodal worms, Ascaridia galli was more predominant in autumn, however, Heterakis gallinarum was recovered in spring, summer and winter. Subulura brumpti was recorded in spring and winter. Trichostrongylus tenuis was only detected in spring. Furthermore, Epomidiostomum uncinatum was only found in autumn (Table 5). Interestingly, the worm burden varied according to the season. The highest worm burden was recorded for both of Amoebotaenia cuneata (24 worms/bird) and A. galli (67 worms/bird) (Table 5). Table 5. The seasonal prevalence of recovered helminth species in ducks.
No.: Number of infected ducks; W.B.: Worm burden; % : Percentage of infection
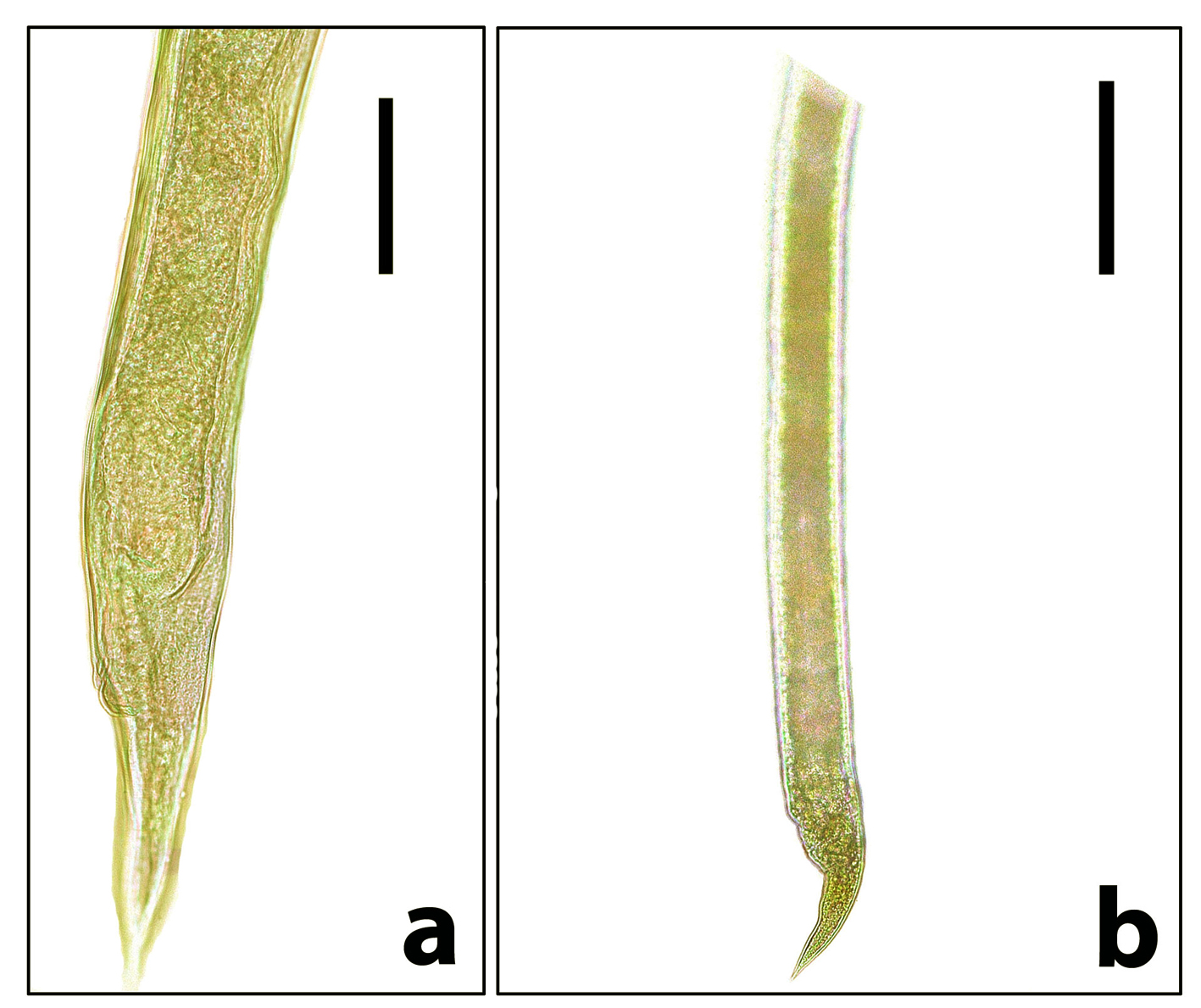
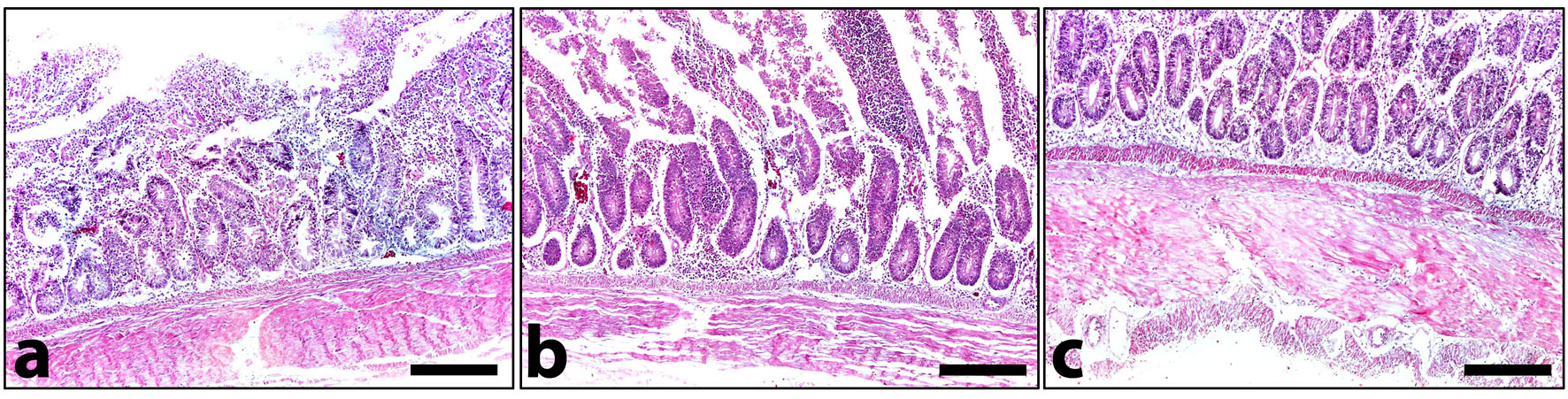
Fig. 4. Stomach and other intestinal nematodes revealed from examined domestic ducks. a) Posterior end of adult female Trichostrongylus tenuis. Note that it gradually narrows to the tip of the tail. Scale bar= 100 µm. b) Posterior end of adult female Epomidiostumum sp. Note that it narrows suddenly towards the tip. Scale bar= 200 µm. Histopathologically, microscopic lesions consisted of severe diffuse degenerative changes and necrosis of intestinal mucosa as well as shortening of intestinal villi could be detected (Fig. 5 a). Congestion of the submucosal blood vessels that filled with numerous nucleated erythrocytes (Fig. 5 b) extravasating from blood vessels into the intestinal mucosa, submucosa and tunica muscularis. The muscular layer had severe degenerative changes associated with focal hyalinosis in certain areas (Fig. 5 c). Diffuse leukocytic infiltration was common.
Fig. 5. A cross section in the small intestine of infected domestic ducks. a) Severe degenerative changes and necrosis of intestinal villi associated with severe submucosal leukocytic infiltration. b) Severe degenerative changes of the lining epithelium, marked leukocytic infiltration and congestion of submucosal blood vessels. c) Hyalinosis of the muscular layer. HE. Scale bar= 200 µm for all parts. |
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
Discussion |
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
The present investigation revealed that the prevalence of intestinal helminthosis in ducks was 13.92% in Beni-Suef, Egypt. Such prevalence closely related to that reported by Mahdy (1988) (12.5%) in Giza, Egypt and higher than that revealed by AbouLaila et al. (2011) (4.54%) in Behera, Egypt and Adang et al. (2014) (4.7%) in Nigeria. The current higher prevalence might be due to the environmental conditions appeared to be favorable for the survival of eggs and the development of insects (hymenopteras like ants, wasps and bees) which serve as an intermediate hosts. Oppositely, Farjana et al. (2008) and Adejinmi and Oke (2011) recorded higher infection rates in Mexican ducks (96.7% and 65.4%, respectively). The higher prevalence might be associated with the free range system of management and rearing of village ducks as well as the amphibious habits of ducks exposing them to greater risk of parasitism (Shah-Fischer and Say, 1989). The present study recovered eight species of tapeworms and five species of nematodes, with a higher prevalence of tapeworm infections rather than nematodes. Such finding agreed with that revealed by Adang et al. (2014), who recovered 7 species of helminths from ducks; 6 tapeworms and one nematode. Meanwhile, Paul et al. (2015) reported 7 species of nematodes and 3 species of tapeworms. On the other hand, Farjana et al. (2004) recovered 17 species consisted of 11 trematodes, 4 tapeworms and two nematodes. Moreover, Muhairwa et al. (2007) recovered 14 helminth species; 12 nematodes and 2 tapeworms. The discrepancy in the prevalence and intensity of helminths could be due to the availability of the infective stages and intermediate hosts of those helminths in areas where ducks feed. Also, the number of birds examined, age, sex and season are incriminated in the infection. The absolute lack of digenean trematodes in this study coincided with the findings of Permin et al. (1997), Yoriyo et al. (2005), Luka and Ndams (2007) and Muhairwa et al. (2007). Authors attributed such finding to the unavailability of intermediate hosts (snails) in the study area. Currently, the prevalence of tapeworms was 8.26%, which was nearly close to that given by Busta (1980) (7.9%) in Canada, and higher than that detected by AbouLaila et al. (2011) (2.5%) in Egypt and McLaughlin and Burt (1979) (83.1%) in Canada. It might be suggested that the higher prevalence of tapeworms in the study area referred to being that the examined ducks could be more susceptible to tapeworm infections or due to the feeding habits of ducks, their free ranging system and loose management. In the present work, eight species of tapeworms were identified. Raillietina spp., were the predominant species. Those helminths are cosmopolitan and their existence is basically associated with malnutrition in birds (Cheng, 1973; Soulsby, 1982). Their intermediate hosts, beetles and ants, are available and abundant, thus extremely serving an essential feed of ducks. This hypothesis explains the occurrence of Raillietina species in infected ducks. Furthermore, the prevalence of nematodes in the examined ducks was 7.84% with the occurrence of five species. The most prevalent one was Ascaridia galli (5.10%), followed by Heterakis gallinarum (1.76%), Subulura brumpti (0.59%), Trichostrongylus tenuis (0.2%) and Epomidiostomum uncinatum (0.2%). These finding went parallel with findings of Adejinmi and Oke (2011) who observed similar results in Southwestern Nigeria. Although the majority of infected ducks (11.76%) had single infection, mixed infections with two or more species were also encountered (2.16%). Similar findings were previously reported by Adejinmi and Oke (2011). Kennedy (1975) reported that the availability of certain food at a particular time might detect the infection, either single or mixed. The higher prevalence of single infections recorded in this study might be referred to the sequence of the parasite invasion, briefly, the first parasite gain access the host, may acquire higher habitats and establishment (Muhairwa et al., 2007; Yousuf et al., 2009). The seasonal variation influencing helminthosis in ducks was previously reported (Birova et al., 1990; Panda et al., 1996; McJunkin et al., 2003). Relatively higher prevalences with helminth parasites were observed in rainy seasons, followed by summer and winter (Anisuzzaman et al., 2005). In the current investigation, the highest peak of infection was found in autumn and summer with a less abundance in winter. Such finding might be attributed to being the fact that insects and other invertebrates, the food of birds which harbor the intermediate hosts of those helminths, are more abundant in hot climates. It is worthy to mention that Epomidiostomum uncinatum has been reported from one mallard duck. Mohammad (2015) reported such nematode from a wide range of hosts. Moreover, it was reported from the mallard Anas platyrhynchos in central Iraq by Mohammad and Al-Moussawi (2011), from the marbled duck, Marmaronetta angustriostris (Mohammad, 2014), and from the shoveler, Anas clypeata (Al-Moussawi, 2014). Histopathologically, rare is reported about helminthosis-induced pathological lesions in domestic ducks. Currently, degenerative changes and necrosis of intestinal mucosa including the intestinal villi were evident. Moreover, extravasation of blood vessels into the entire epithelial lining occurred. Authors suggested that the presence of various tapeworm species which damage the host cells via their armed suckers and rostellum might be the principal cause. As a result of the host defense mechanism, inflammatory reactions with mononuclear/polymorphnuclear leukocytes infiltrations were easily recognized (Brener et al., 2006). Meanwhile, the infection with Raillietina and Hymenolepis spp. induces a catarrhal inflammation of the intestinal mucosa (Islam et al., 1988). Although helminthiasis in ducks often with mild clinical symptoms, pathological lesions were observed in severe infections, particularly when the tissue-invading parasites were involved. |
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
Conclusion Due to the occurrence of a variety of helminths in domestic ducks in Beni-Suef, Egypt, further works should be simultaneously done to investigate both endo- and ectoparasites in ducks and other aquatic birds in the same districts and related areas with the same habits and environmental conditions. Moreover, molecular studies are requested to explore the appropriate existence of helminths, particularly tapeworms. |
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
Conflict of Interests |
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
Authors declare that there is no conflict of interest. |
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
References |
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
AbouLaila, M., El-Bah, N., Hilali, M., Yokoyama, N., Igarashi, I., 2011. Prevalence of the enteric parasites of ducks from Behera governorate, Egypt. Journal of Protozoology Research 21, 36-44. Adang, K.L., Asher, R., Abba, R., 2014. Gastrointestinal helminths of domestic chicken Gallus domesticus and ducks Anas platyrhynchos slaughtered at Gombe main markets, Gombestate, Nigeria. Asian Journal of Poultry Science 8, 32-40. Adejinmi, J.O, Oke, M., 2011. Gastro-intestinal parasites of domestic ducks (Anas platyrhynchos) in Ibadan Southwestern Nigeria. Asian Journal of Poultry Science 5, 46-50. Al-Moussawi, A.A., 2014. Stomach nematodes of the Shoveler Anas clypeata Linnaeus, 1758 (Anseriformes: Anatidae) wintering in Iraq. Bulletin of the Iraq Natural History Museum 13, 27-34. Ahmed, N.E., Loubna, M.A., El-Madawy, R.S., Toulan, E.I., 2013. Studies on helminthes of poultry in Gharbia Governorate. Benha Veterinary Medical Journal 25, 139-144. Anisuzzaman, Alim, M.A, Rahman, M.H, Mondal, M.M.H., 2005. Helminth parasites in indigenous ducks: Seasonal dynamics and effects on production performance. Journal of the Bangladesh Agricultural University 3, 283-290. Bancroft, J.D., Stevens, A., 1996. Theory and practice of histological techniques. 4th edn. New York, Churchill Livingstone. Birova, V., Macko, J.K., Spakulova, M., 1990. Seasonal dynamics of the invasion cycle of nematodes and acanthocephalans in the wild (Anas platyrhynchos L.) and domestic duck (Anas platyrhynchos f. dom.). Helminthologia 27, 291-301. Brener, B. Tortelly, R., Menezes, R.C., Muniz-Pereira, L.C., Pinto, R. M., 2006. Prevalence and pathology of the nematode Heterakis gallinarum, the trematode Paratanaisia bragai, and the protozoan Histomonas meleagridis in the turkey, Meleagris gallopavo. Memórias do Instituto Oswaldo Cruz 101, 677-681. Busta, J., 1980. Helminths in broiler geese fattened in runs. Veterinary Medicine (Praha) 25, 717-723. Cheng, T., 1973. General Parasitology. Academic Press, New York, San-Francisco, pp: 434-936. Cooper, J.E., 1984. A veterinary approach to pigeons. Journal of Small Animal Practice 25, 505-516. Cole, R.A., Friend, M., 1999. Miscellaneous parasitic diseases. In: Friend M, Franson, J.C. (eds.), Field Manual of Wildlife Diseases. U.S. Geological Survey, Biological Resources Division, National Wildlife Health Center, Madison, Wisconsin, pp. 249-262. Dudley, J.P., Hoberg, E.P., Jenkins, E.J., Parkinson, A.J., 2015. Climate change in the North American Arctic: a one health perspective. EcoHealth 12, 713-725. El-Dakhly, Kh. M., El-Nahass, E., Uni, S., Tuji, H., Sakai, H., Yanai, T., 2012. Levels of infection of gastric nematodes in a flock of great cormorants (Phalacrocorax carbo) from Lake Biwa, Japan. Journal of Helminthology 86, 54-63. Farjana, T., Alim, M.A., Das, P.M., Mondal, M.M.H., 2004. Helminth infection in ducks at free range and semi intensive farming in two districts of Bangladesh. Bangladesh Veterinary Journal 38, 125-134. Farjana, T., Islam, K. R., Mondal, M. M. H., 2008. Population density of helmints in ducks: effects of host’s age, sex, breed and season. Bangladesh Journal of Veterinary Medicine 6, 45-51. Gicik, Y., Arslan, M.Ö., 2003. The prevalence of helminths in the alimentary tract of geese (Anser anser domesticus) in Kars District, Turkey. Veterinary Research Communication 27, 391-395. Harlin, R.W., 1994. Pigeons. Veterinary Clinics of North America: Small Animal Practice 24, 157-173. Harrison, C., Greensmith, A., 1993. Birds of the World. Dorling Kindersly, London, UK. Islam, M.R., Shaikh, H., Baki, M.A., 1988. Prevalence and pathology of helminth parasites in domestic ducks of Bangladesh. Veterinary Parasitology 29, 73-77. Kennedy, C.R., 1975. Ecological Animal Parasitology, John Wiley and Sons, Oxford UK, p. 163. Luka, S.A., Ndams, I.S., 2007. Gastrointestinal parasites of domestic chicken Gallus-gallus domesticus Linnaeus 1958 in Samara, Zaria Nigeria. Science World Journal 2, 27-29. Mahdy, O.A., 1988. Studies on parasitic worms infesting chickens and ducks in Giza governorate, Egypt. M.V.Sc. Thesis Fac. Vet. Med. Cairo University, Egypt. Margolis, L., Esch, G.W., Holmes, J. C., Kuris, A. M., Schad, G. A., 1982. The use of ecological terms in Parasitology. (Report of an AdHoc Committee of the American Soceity of Parasitology). Journal of Parasitology 68, 131-133. McJunkin, J.W., Applegate, R.D., Zelmer, D.A., 2003. Enteric heminths of juvenile and adult wild turkeys in Easter Kansas. Avian Diseases 47, 1481-1485. McLaughlin, J.D., Burt, M.D., 1979. A survey on the intestinal helminth of water fowl from New-Brunswick, Canada. Canadian Journal of Zoology 57, 801-807. Mohammad, M. K., 2014. The parasitic fauna of the marbled teal Marmaronetta angustirostr is (Menetries, 1823) L. Reichenbach 1853 in the middle of Iraq. International Journal of Recent Scientific Research 5, 54-56. Mohammad, M.K., 2015. The parasitic fauna of the Ferruginous duck Aythyanyroca (Güldenstädt, 1770) collected in central Iraq. International Journal of Advanced Research in Biological Sciences 2, 62-65. Mohammad, M. K., Al-Moussawi, A. A., 2011. Prevalence and infection rate of three gizzard nematodes in the mallard Anas platyrhynchos L., 1758 Collected in AlDiwaniya and Diyala Provinces, Central Iraq. Ibn Al-Haitham Journal for Pure Applied Science 24, 15-24. Muhairwa, A.P., Msoffe, P.L., Ramadhani, S., Mollel, E.L., Mtambo, M.M.A., Kassuku, A.A., 2007. Prevalence of gastro-intestinal helminths in free-range ducks in Morogoro Municipality, Tanzania. Livestock Research for Rural Development. Volume 19, Article #48. Retrieved October 29, 2019, from http://www.lrrd.org/lrrd19/4/muha19048.htm Olsen, G.H., 2009. Bacterial and parasitic diseases of Anseriformes. Veterinary Clinics of North America: Small Animal Practice 12, 475-490. Panda, N.K., Panda, D.N., Misra, S.C., Panda, M.R., 1996. Effect of season and age on the prevalence of helminthic infections in ducks at Bhubaneswar, Orissa, India, Journal of Veterinary Parasitology 10, 69-73. Paul, B.T., Lawal, J.R., Ejeh, E.F., Ndahi, J.J., Peter, I.D., Bello, A.M., Wakil, Y., 2015. Survey of helminth parasites of free range Muscovy ducks (Anas platyrynchos) slaughtered in Gombe, North Eastern Nigeria. International Journal of Poultry Science 14, 466-470. Permin, A., Magwisha, H., Kassuku, A., Nansen, P., Bisgaard, M., Frandsen, F., Gibbons, L., 1997. A cross-sectional study of helminths in rural scavenging poultry in Tanzania in relation to season and climate. Journal of Helminthology 71, 233-240. Poulin, R., 2006. Global warming and temperature-mediated increases in cercarial emergence in trematode parasites. Parasitology 132, 143-151. Radfar, M.H., Fathi, S., Asl, E.N., Dehaghi, M.M., Seghinsara, H.R., 2011. A survey of parasites of domestic pigeons (Columba livia domestica) in South Khorasan, Iran. Veterinary Research 4, 18-23. Rzad, I., Sitko, J., Kavetska, K., Kalisinska, E., Panicz, R., 2013. Digenean communities in the tufted duck (Aythyafuligula L., 1758) and greater scaup (A. marila L., 1761) wintering in the north-west of Poland. Journal of Helminthology 87, 230-239. Shah-Fischer, M., Say, R., 1989. Manual of Tropical Veterinary Parasitology.CAB International: The Technical Center for Agricultural and Rural Cooperation (CTA). Soulsby, E.J.L., 1982. Helminths, Arthropods and Protozoa of Domesticated animals.7th edn., London, Bailliere Tindall. Yamaguti, S., 1961. Systema Helminthum. Vol. III. The nematodes of vertebrates. Part 1. New York, Interscience, p. 680 Yoriyo, K.P., Fabiyi, J.P., Panda, S.M., Adamu, S.U., 2005. Intensities of helminth parasites of free ranging chickens in bauchi and environs. Yankari Journal 2, 135-139. Yousuf, M.A., Anisuzzaman M., Banowary, B., 2009. Gastrointestinal helminths of ducks: some epidemiological and pathological aspects. Journal of the Bangladesh Agricultural University 7, 91-97. Wang, B.W., Jia, X.H., Wu, X.P., Zhang, M.A., Liu, G.L., Sun, X.H., 2004. Research on the epidemiology of goose parasites in Shandong Province. Journal of Laiyang Agricultural College 21, 185-188 (in Chinese). |
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||