|
|
Journal of Advanced Veterinary Research Volume 10, Issue 2, 2020, Pages: 73-80 www.advetresearch.com |
|
|
Morphological and Molecular characterization of Sarcocystis arieticanis from the Heart Muscles of Domestic sheep, Ovis aries, in Qena, Upper Egypt |
||||||||||||||||||||||||||||||||||||||||||||
|
|
||||||||||||||||||||||||||||||||||||||||||||
|
Nermean M. Hussein |
||||||||||||||||||||||||||||||||||||||||||||
|
|
||||||||||||||||||||||||||||||||||||||||||||
|
Zoology Department, Faculty of Science, South Valley University, Qena 83523, Egypt (nermeanmohu@yahoo.com). |
||||||||||||||||||||||||||||||||||||||||||||
|
|
||||||||||||||||||||||||||||||||||||||||||||
|
Received: 16 February 2020; Accepted: 20 March 2020 |
||||||||||||||||||||||||||||||||||||||||||||
|
Abstract |
||||||||||||||||||||||||||||||||||||||||||||
|
|
||||||||||||||||||||||||||||||||||||||||||||
|
This study examined Sarcocystis spp., cyst forming coccidians whose intermediate hosts include sheep, an important source of meat in Egypt. Samples of heart muscles were collected from 110 sheep from different localities in Qena, Upper Egypt, and examined for Sarcocystis spp. by macroscopic evaluation, light microscopy, transmission electron microscopy, and molecular tests. All the sarcocysts found [in 52 of 110 sheep (47.27 %)] were thin-walled (0.32–0.44 µm) and measured 78.56–146 µm in length and 33.12–73 µm in width. The cyst walls possessed irregularly branched protrusions (0.67–1.4 µm × 0.05–0.26 µm) with rounded vesicles at their base that were underlin by a thin layer of ground substance (0.14–0.26 µm). Septa derived from the ground substance divided the cysts into many compartments that contained cystozoites of different developmental stages and fully formed banana-shaped bradyzoites, for which descriptions are provided based on transmission electron microscopy. Comparison of the 18S rRNA sequence of the sarcocysts against those of previously identified species in mammals deposited in GeneBank was performed. The phylogenetic tree clustered the Sarcocystis specimens in this study within the S. arieticanis clade. In addition, the macroscopic Sarcocystis spp. (S. moulei and S. gigantea) aggregated together at some distance from the microscopic Sarcocystis spp. (S. capracanis, S. hiricicanis, S. tenella, and S. arieticanis) regardless of differences in the intermediate host, indicating the importance of Sarcocystis cyst size as a characteristic taxonomic feature. |
||||||||||||||||||||||||||||||||||||||||||||
|
|
||||||||||||||||||||||||||||||||||||||||||||
|
Keywords: Sarcocystis arieticanis, Sheep, Heart, TEM, 18S rRNA, Phylogenetic tree |
||||||||||||||||||||||||||||||||||||||||||||
|
|
||||||||||||||||||||||||||||||||||||||||||||
|
Introduction |
||||||||||||||||||||||||||||||||||||||||||||
|
|
||||||||||||||||||||||||||||||||||||||||||||
|
Sarcocystis spp. are apicomplexan protozoa with an obligatory heteroxenous life cycle, which alternates between an intermediate host and a definitive host (Dubey et al., 1989). They are common parasites of a broad range of vertebrates including mammals, birds, and reptiles (Heydorn and Mehlhorn, 1987; Dubey et al., 1988; Bashtar et al., 1990; Abdel-Ghaffar et al., 1990a; Abdel-Ghaffar et al., 1990b; Abdel-Ghaffar and Al Johany, 2002; Haziroglu et al., 2003; Abdel-Ghaffar et al., 2009; Olias et al. 2010; Xiang et al. 2010; Mandour et al., 2011; Morsy et al., 2011; Abdel-Baki et al., 2012). Sheep (Ovis aries) are the intermediate hosts of at least six named species of Sarcocystis, including S. tenella (syn. S. ovicanis), S. gigantea (syn. S. ovifelis), S.arieticanis, S. medusiformis, S. mihoensis, and S.microps (Hu et al., 2017), all of which are morphologically differentiated based on their sarcocyst wall ultrastructure. S. tenella and S. arieticanis produce microscopic sarcocysts transmitted by canids, while S. gigantea and S. medusiformis produce macroscopic cysts transmitted by felids (Dubey et al., 2016). The remaining two species, S. mihoensis and S. microps, transmitted by canids, are unusual or rare. S. mihoensis, reported only from Japan, produces macroscopic sarcocysts (Saito et al., 1997), while S. microps, reported only once from China, produces microscopic sarcocysts (Wang et al., 1988). Ultrastructural study of the cyst wall has been used for the identification of Sarcocystis spp. isolated from different animals by many researchers (Mehlhorn and Heydorn, 1979; Dubey et al., 1989; Abdel-Ghaffar et al., 2009; Jehle et al., 2009). Infection with Sarcocystis may prove fatal to the host (Tenter, 1995; Fayer, 2004). Haziroglu et al. (2003) recorded S. arieticanis from cardiac muscles of sheep and were able to describe its wall with the use of transmission electron microscopy (TEM), while Al -Quraishy et al. (2014) and Mehlhorn et al. (2014) detected S. arieticanis from the heart muscles of domestic sheep based on both light and electron microscopy. Bittencourt et al. (2016) observed S. tenella and S. arieticanis in tongue muscles of sheep in Brazil. Hussein et al. (2018) identified S. tenella in esophagus muscles of sheep in Qena, Upper Egypt using transmission electron microscopy (TEM) and molecular techniques. As part of an ongoing study on Sarcocystis spp. infecting sheep in Qena, Upper Egypt (Hussein et al., 2018), a detailed morphological redescription and molecular characterization of S. arietcanis collected from sheep heart muscles are presented in this study. |
||||||||||||||||||||||||||||||||||||||||||||
|
|
||||||||||||||||||||||||||||||||||||||||||||
|
Materials and methods |
||||||||||||||||||||||||||||||||||||||||||||
|
Sampling Specimens were collected from the heart muscles of 110 sheep from different slaughterhouses in Qena, Upper Egypt (93 males and 17 females; aged 6 months to 3 years) during the period from April 2018 to March 2019. The tissues were transported to the laboratory and were examined for Sarcocysts. Ethical approval was granted by the research and Ethics committee of the Faculty of Science, South Valley University for animal use and collecting permits. Macroscopic examination Macroscopic examination was performed by using a hand lens whereas muscle samples was cut into smaller pieces for the presence of macrocysts, which appeared whitish with different shapes according to Lam et al. (1999). Microscopic examination of fresh tissue Small pieces of heart muscles from each sample were pressed between two glass slides and examined using a light microscope according to Hu et al. (2017). Histological examination Small pieces of infected heart muscle were fixed in 10% buffered formalin, prepared for 5-µm paraffin sections, stained with hematoxylin eosin (HE) stain for histological examination and photographed according to Mandour et al. (2011). Transmission electron microscopy Small pieces of infected heart muscles of one specimen were fixed in 3% cold glutaraldehyde solution in sodium cacodylate buffer 0.1 M (pH 7.4) for 24 h, washed in sodium cacodylate buffer 0.1 M (pH 7.4), postfixed in 1% osmium tetroxide, dehydrated with ethyl alcohol, then embedded in resin. Semi-thin sections were examined by light microscopy. Ultra-thin sections were examined under JEM 100 CXII electron microscope at 80 KV and photographed using CCD digital camera Model XR-41 (Hussein et al., 2018), then the developmental stages were drawed for the first time in present study. Molecular characterization Infected heart muscle tissues were cut into small pieces and were preserved in ethyl alcohol 95% at 20 °C for total genomic DNA extraction. DNA was extracted using a DNA Extraction Kit (Qiagen, DNeasy® QIAamp DNA Mini, and Blood Mini Kit). PCR amplifications were performed with a total volume of 50 μl consisting of 2 μl of DNA template, 25 μl PCR master mix of concentration ( 1× PCR buffer, 16 mM (NH4)2SO4, 67 mM Tris–HCl at pH 8.8, 2 mMMgCl2, 200 μM of each dNTP, 2μM taq DNA polymeraze), 0.7 mM each primer and 0.7 unit BIOTAQ™ DNA polymerase (Bioline Ltd.), made up to 50 μl with Invitrogen™ ultraPURE™ distilled water. Partial fragments of the 18S rRNA gene (~700 bp) were amplified using the primers 2L (forward: 5ʹ-CGCAAATTACCCAATCCTGA-3ʹ) and 3H (reverse: 5ʹ-ATTTCTCATAAGGTGCAGGAG-3ʹ) under the thermocycling conditions specified by Yang et al. (2002) as follows: denaturation at 95 °C for 4 min followed by 35 cycles of amplification (94 °C for 30 s, 59 °C for 30 s, 72 °C for 1 min) and a final extension step at 72 °C for 5 min. PCR amplicons were purified using a QIAamp PCR Purification Kit (QIAGEN) according to the manufacturer’s protocol and were sequenced directly using an ABI Prism Big Dye Terminator V.3.1 Cycle Sequencing Kit on an Applied Biosystems 3500 automatic Genetic Analyzer following the manufacturer’s recommendations using the same PCR primers. Forward and reverse PCR amplicon reads were used for sequence assembly and editing as recommended by Blasco-Costa et al. (2016). GeneStudio™ Professional Edition V. 2.2.0.0 was used to assemble sequences, edit contiguous sequences, and generate consensus sequences. Newly generated partial 18S rRNA sequences were aligned with sequences of species within the genus Sarcocystis and selected outgroup taxa available in GenBank. Sequences were aligned using MUSCLE (Edgar 2004) as implemented in MEGA7 (Kumar et al., 2016) and trimmed to a point where the majority of sequences had started and finished. Two sets of data were obtained, phylogenetic analysis was performed for each one with maximum likelihood (ML), and a best nucleotide substitution fit model was determined using an implemented tool in MEGA7 (Nei and Kumar 2000) where the Akaike Information Criterion indicated that (T92+G+I) was the best estimator for ML settings in both data sets. The bootstrap consensus tree was inferred from 1000 replicates and branches corresponding to partitions reproduced in less than 50% bootstrap replicates were collapsed. The percentage of replicate trees in which the associated taxa clustered together in the bootstrap test is shown next to the branches except for bootstrap values less than 50%. Initial tree(s) for the heuristic search were obtained automatically by applying Neighbor-Join and BioNJ algorithms to a matrix of pairwise distances estimated using the Maximum Composite Likelihood approach and then selecting the topology with superior log likelihood value. |
||||||||||||||||||||||||||||||||||||||||||||
|
|
||||||||||||||||||||||||||||||||||||||||||||
|
Results |
||||||||||||||||||||||||||||||||||||||||||||
|
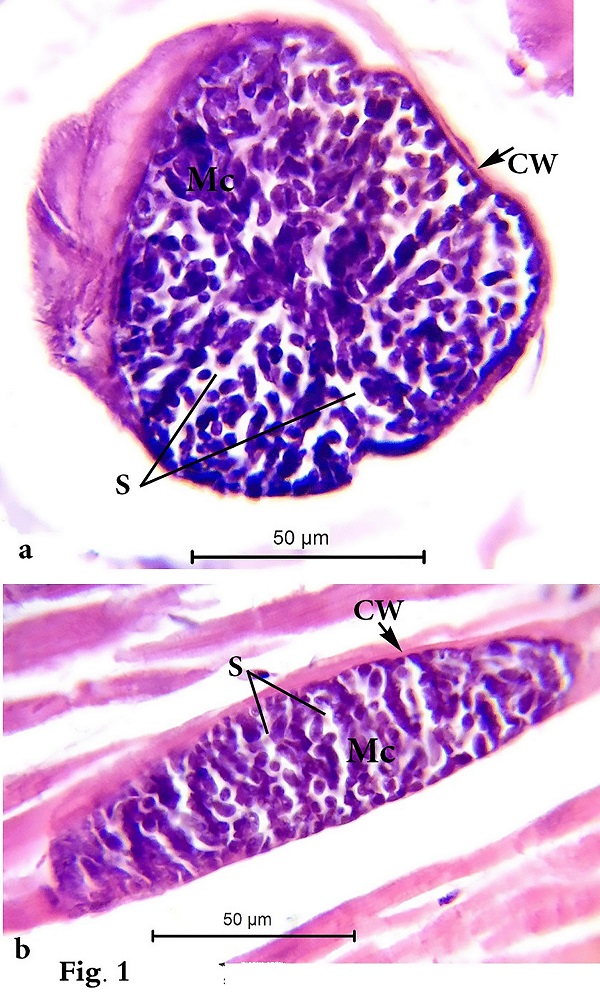
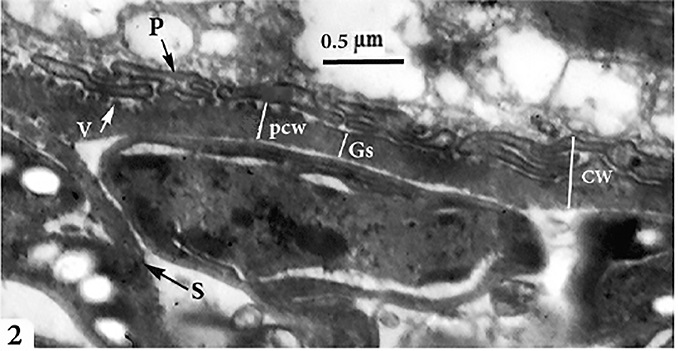
Morphological data Macroscopic evaluation revealed no infection with macroscopic sarcocysts in the heart muscles of the domestic sheep. Light microscopy revealed the presence of thin-walled septate microscopic sarcocysts in 52 out of 110 sheep (47.27%), comprising 41 of 93 males (44.08%) and 11 of 17 females (64.70%). The rate of infection in sheep aged over 1 year was greater than that in sheep aged under 1 year. Infection was found in 32 of 58 males (55.17%) aged over 1 year, and in 9 of 35 males (25.71%) aged under 1 year. Infection was found in 9 of 11 females (81.8%) aged over 1 year, and in 2 of 6 females (33.33%) aged under 1 year (Tables 1 and 2). Cyst length was 78.56–146.21µm, and cyst width was 33.12–73.54 µm, (n = 35) (Fig. 1). TEM showed that the cyst wall was thin (0.32–0.44 µm) and contained horizontally branched protrusions (0.67–1.40 × 0.05–0.26 µm; n = 40) with rounded vesicles (0.051–0.066 × 0.046–0.066; n = 40) at their base underlain by a thin layer of ground substance (0.14–0.26 µm) located directly under the primary cyst wall (Figs 2, 4). Several septa derived from the granular layer divided the cyst into many compartments that contained an abundance of cystozoites at different developmental stages.
Fig. 1. Photomicrographs of (a) transverse and (b) longitudinal histological paraffin sections of heart muscles of sheep infected with Sarcocystis sp. stained with H&E showing the cyst wall (Cw), merozoites (Mc), and septa (S) that divide the cyst into many chambers.
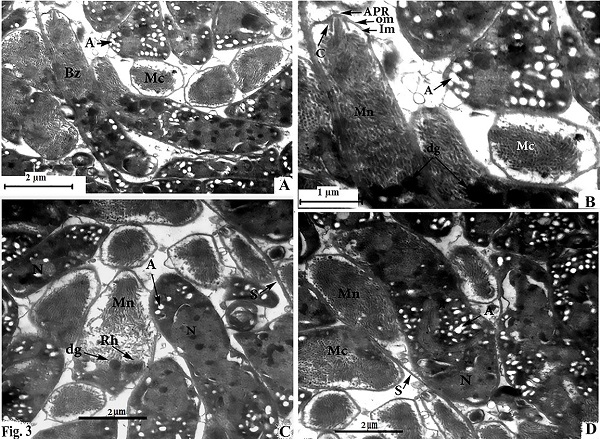
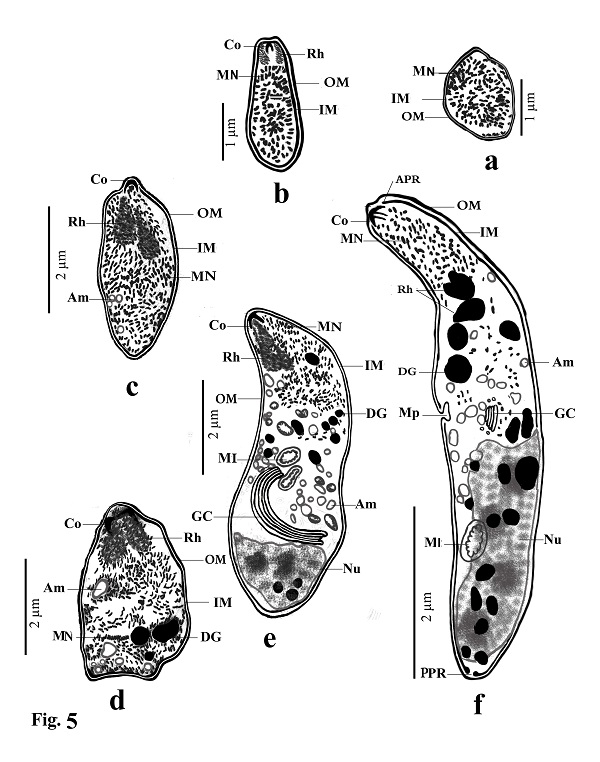
Fig. 2. Transmission electron micrographs of cross sections through a sarcocyst in cardiac muscles of sheep The cyst wall (CW), irregular horizontally branched protrusions (P), ground substance (GS) that extend into septa (S), and vesicles in the base of protrusions (V). Developmental stages of bradyzoites The youngest stage was ovoid with a pointed anterior end (1.2–1.8 × 1.7–2.0 µm; n = 20) filled with micronemes; no other organelles were seen (Figs 3A, C, D and 5a). The second stage was pyriform in shape (0.71–0.86 × 2.0–2.5 µm; n = 20), with a small conoid in its anterior end and a large number of micronemes (Figs 3C, D and 5b). The third stage was also pyriform but larger (1.4–1.8 × 3.1–3.8 µm; n = 20) with conoids, rhoptries, amylopectin and many micronemes (Figs 6, 8c). The fourth stage was irregularly-shaped (2.0–2.4 × 3.4–3.8 µm; n = 20) with conoids, rhoptries, amylopectin, and dense granules, but with fewer micronemes than in all the previous stages (Figs 3C and 5d). The fifth stage was elongate banana-shaped (2.0–2.4× 5.2–7.1 µm; n = 20) containing a conoid, rhoptries, micronemes, amylopectin, dense granules, a nucleus and mitochondria (Figs 3D and 5e). The sixth stage, fully formed banana-shaped bradyzoites, was surrounded with an inner and an outer membrane (1.5–2.3 × 6 .1–8.5 µm; n = 20) containing all the organelles characteristically found in other Sarcocystis spp. (Figs 3, 5f).
Fig. 3. electron micrographs of cross sections through a sarcocyst in cardiac muscles of sheep A: Fully formed bradyzoites with apical polar ring (APR), conoid (C), outer membrane (OM), inner membrane (IM), micronemes (MN), dense granules (dg), and several amylopectin (A), developing bradyzoite (Mc), and nucleus (N) B: Enlarged anterior part of fully formed bradyzoites. C and D Fine globular metrocyte with micronemes (MN), rhoptries (Rh), dense granules (dg), nucleus (N), and several amylopectin (A).
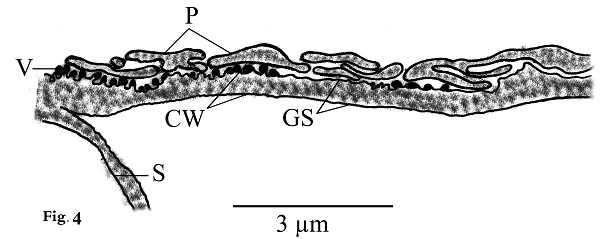
Fig. 4. Line drawing of thin cyst wall (CW) of present Sarcocystis arieticanis. Note protrusions (P), vesicles (V), smooth ground substance (GS), and septa (S).
Fig. (5a–f). Line drawings of different developmental stages of cystozoite of present Sarcocystis arieticanis from heart muscles of domestic sheep. Note outer membrane (OM), inner membrane (IM), micronemes (MN), conoid (CO), rhoptries (Rh), anterior polar ring (APR), posterior polar ring (PPR), dense granules (DG), amylopectin (Am), micropore (MP), mitochondria (MI), Golgi complex (GC) and nucleus (N). Table 1. Number of male and female sheep examined and the rate of infection with microscopic Sarcocystis sp.
Table 2. Number of sheep examined and the rate of infection by age
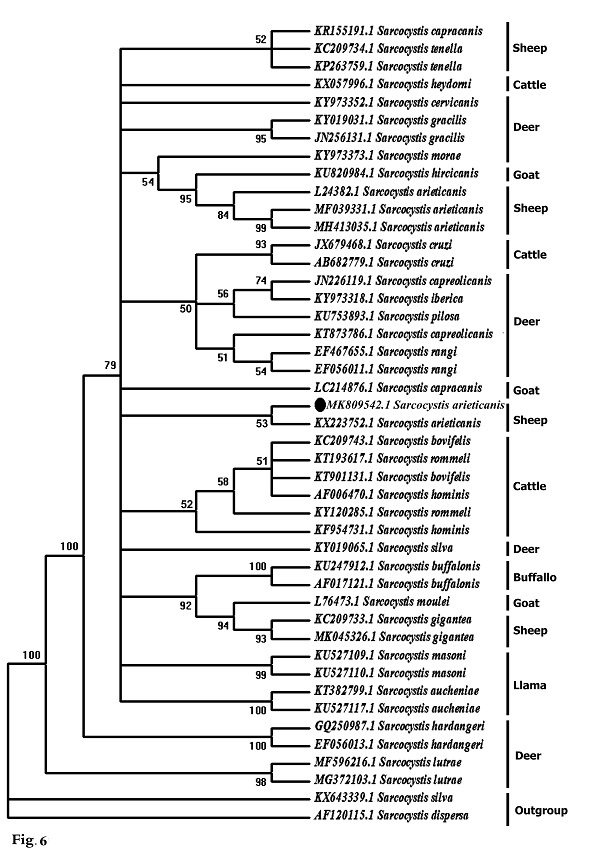
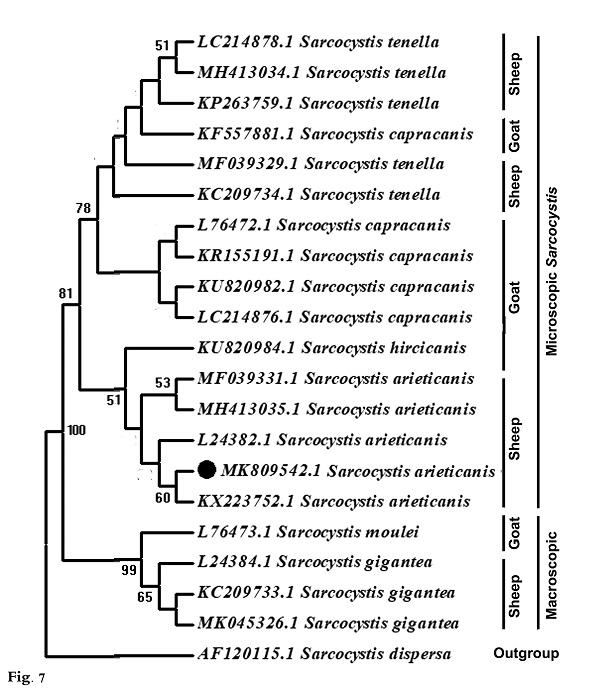
Molecular data A partial region of the 18S rRNA gene of the Sarcocystis sp. observed in this study was sequenced (698 bp) and recorded in GenBank under accession number MK809542. This sequence was compared to other Sarcocystis taxa and aligned with two sets of data representing all the available and appropriate species, for which sequence data were available. In the first data set, the present sequence was compared to other Sarcocystis taxa known to infect herbivorous mammals, and 42 sequences were obtained representing 24 different Sarcocystis taxa: 10 from deer, 4 from cattle, 1 from buffalo, 1 from both cattle and buffalo, 2 from llamas, 3 from goats, and 3 from sheep (Fig. 6). The second data set included all Sarcocystis taxa known infect only sheep and goats where both are found together in the same habitat and geographical area, sharing the same food. Nineteen sequences were obtained representing 6 different Sarcocystis taxa: 3 from goats (S. moulei, S. capracanis., S. hiricicanis) and 3 from sheep (S. tenella, S. arieticanis, S. gigantea) (Fig. 7). For outgroup comparisons, 1 sequence from Sarcocystidae, the avian S. dispersa with accession number AF120115, was used in the second sequence data set.
Fig. 6. Phylogenetic tree for selected members of Sarcocystis spp. infecting herbivorous mammals based on 18S rRNA sequences and inferred by the maximum likelihood (ML) method. The analysis involved 43 nucleotide sequences whose GenBank accession numbers are shown behind the taxon names. Numbers beside each branch show bootstrap values; values below 50% are not shown. Phylogenetic analysis of the first data set clarified clustering of S. capracanis and S. tenella in a well-supported clade (52%) and also the clustering of S. moulei and S. gigantea in a strongly-supported clade (94%). In the second data set phylogenetic analysis, Sarcocystis from both sheep and goats formed a monophyletic clade that excluded the outgroup taxon. S. gigantea and S. moulei aggregated in a strongly-supported clade (99%) which itself was a sister to a strongly-supported clade (81%) containing other Sarcocystis taxa: S. arieticanis, S. hiricicanis, S. capracanis, and S. tenella. The present S. arieticanis resolved within a well-supported clade (56%) representing taxa of S. arieticanis. Sarcocystis hiricicanis resolved in a basal position to all S. arieticanis taxa forming a well-supported clade (51%) which in turn was a sister to the strongly-supported S. capracanis/S. tenella clade (78%).
Fig. 7. Phylogenetic tree for Sarcocystis taxa known to infect sheep and goats only based on 18S rRNA sequences and inferred by the maximum likelihood (ML) method. The analysis involved 20 nucleotide sequences whose GenBank accession numbers are shown behind the taxon names. Numbers beside each branch show bootstrap values; values below 50% are not shown. |
||||||||||||||||||||||||||||||||||||||||||||
|
|
||||||||||||||||||||||||||||||||||||||||||||
|
Discussion |
||||||||||||||||||||||||||||||||||||||||||||
|
Sarcocystis spp. are among the most common parasites in domestic ruminants, and some of them can generate significant economic losses when causing clinical and subclinical disease. Up till now, at least six species of Sarcocystis have been named in sheep; however, only four (S. tenella, S. arieticanis, S. gigantea, and S. medusiformis) have been frequently found in different areas (Dubey et al., 2016). Sarcocystis spp. have the ability to invade different organs of their intermediate host (Bashtar et al., 1992; Abdel-Ghaffar et al., 2009). Two Sarcocystis spp. with microscopic cysts (S. tenella and S. arieticanis) and two with macroscopic cysts (S. gigantea and S. medusiformis) infect sheep according to Dubey et al. (1989). Differentiation between the microscopic species was based on the morphological criteria described by Levine (1985) and Dubey et al. (1989). The present study revealed that the rate of infection in heart muscles of sheep with microscopic S. arieticanis was 47.27%, comparing with results of El-Morsey et al. (2019) in Egypt that was 38.8% and Al Quraishy et al. (2014) that was, 40-53%. in different localities of K.S.A., and less than in Kanming City, China (91.9%) as reported by Hu et al. (2017). According to the available literatures, results of this study refered to the rate of infection according to gender and age that was not reported in all the previous studies. The ultrastructure of the cyst wall in the present specimens was “type 7” according to the classification of cyst walls reported by Dubey et al. (2016). In this study, Sarcocystis sp. was microscopic and septated and had a thin cyst wall with horizontally branched finger-like protrusions that appeared parallel to the surface; they contained vesicles at their base, and the cyst wall protrusions did not have dome-shaped bases. This appearance is similar to that seen in the electron micrographs of Heydorn and Melhorn (1987); Odening et al. (1995); Haziroglu et al. (2003) and Al Quraishy et al. (2014). There were some morphological differences ( e.g., size and shape of protrusions) between the present specimens and those from previous reports that described S. arieticanis from organs other than heart muscle, including the esophagus, diaphragm and tongue muscles of sheep (Dubey et al., 1988; Bittencourt et al., 2016; Hu et al., 2017; El Morsey et al., 2019). Those reports described the presence of a dome-shaped base in the cyst wall protrusions that was absent in the present specimens. This morphological characteristic may be related to the nature of the host muscles and cyst age; protrusions of the cyst walls may be folded in older cysts (Haziroglu, 2003), the morphology may change with age (Dubey et al., 1989) and the cyst walls may differ depending on the muscle tissues in which they are developing (Haziroglu et al., 2003). The ultrastructure of the present cysts was similar to that of Sarcocystis spp. in different Caprinae; for example, S. arieticanis in wild sheep (O. musimon), S. hiricicanis in goats (Hu et al., 2016), S. hiricicanis/arieticanis-like in blue sheep (Pseudois nayaur), Japanese serow (Capricornis crispus), and musk ox (Ovibos moschatus) (Odening et al., 1996). The cyst wall in specimens from the current study was thinner and differed from that in S. capracanis by having a thick- radially striated wall with finger-like projections (Hu et al., 2016; Morsy et al., 2011). The present Sarcocystis sp. differed from microscopic S. tenella from the esophagus and heart muscles of sheep, with the latter having palisade-like protrusions and unusual plaque structures in the tips of the villi (Hu et al., 2017; Hussein et al., 2018); it also differs from the microscopic thick-walled sarcocysts (S. capracanis) in goats (Mahran, 2009). The phylogenetic tree (Fig. 7) shows that different species of sheep Sarcocystis did not form a single cluster and were not necessarily clustered in closely related clades; the same was observed for the different species of goat Sarcocystis. In fact, the sheep Sarcocystis (S. arieticanis, S. tenella, and S. gigantean) aggregated with the goat Sarcocystis (S. hiricicanis, S. capracanis, and S. moulei respectively) forming three well-supported clades: S. capracanis/S. tenella, S. arieticanis/S. hiricicanis, and S. gigantea/S. moulei. Furthermore, S. gigantea/S. moulei clustered at a distance from S. arieticanis, S. tenella, S. hiricicanis, and S. capracanis in a separate clade. Morphologically, both S. gigantea and S. moulei were found to belonging to the macroscopic Sarcocystis, whereas S. hiricicanis, S. capracanis, S. arieticanis, and S. tenella were seen to belong to the microscopic Sarcocystis (Dubey et al., 2016). Thus, the separation of S. gigantea and S. moulei from the S. capracanis/S. tenella and S. arieticanis/S. hiricicanis clades can be attributed to their micrscopic cyst size, inferring that cyst size is a strong differentiating morphological feature that should be considered first when classifying Sarcocystis spp. In both phylogenetic trees S. arieticanis and S. hiricicanis resolved as very closely related (Figs, 6 and 7). Both S. arieticanis and S. hiricicanis are extremely similar, with no critical feature observed for distinguishing between them; they may be distinguished only by intermediate host specificity (Hu et al., 2016) as shown experimentally by unsuccessful transmission experiments with S. arieticanis in goats (Heydorn, 1985; Dubey et al., 1989) and with S. capracanis and S. hiricicanis in sheep (Balbo et al., 1988; Dubey et al., 1989). Furthermore, sheep and goats (both of which have the same habitat, food type, and feeding manner) have been reported as harboring both Sarcocystis spp. (Dubey and Rosenthal, 2013; Formisano et al., 2013; Hu et al., 2016. Accordingly, the lack of distinctive characteristics for distinguishing between S. arieticanis and S. hiricicanis as well as their very close phylogenetic relationship (and allied to their hosts’ shared food type and feeding habitats), it is not satisfactory to recognize S. hiricicanis as a distinct species. S. hiricicanis is highly likely just a synonym for S. arieticanis, through additional molecular characterization and further detailed morphological studies are recommended. |
||||||||||||||||||||||||||||||||||||||||||||
|
|
||||||||||||||||||||||||||||||||||||||||||||
|
Conclusion To the author's best knowledge, the current investigation is the first to demonstrate the presence of the microscopic Sarcocystis arieticanis in heart muscles of domestic sheep slaughtered for human consumption in Upper Egypt, with evidence from ultrastructural and molecular identification of isolated sarcocysts and description of the different developmental stages of bradyzoites using TEM. |
||||||||||||||||||||||||||||||||||||||||||||
|
|
||||||||||||||||||||||||||||||||||||||||||||
|
Acknowledgments |
||||||||||||||||||||||||||||||||||||||||||||
|
Thanks to Yasser Farhat Mahmoud Karar Assistant Lecturer of Zoology, Zoology Department, Faculty of Science, South Valley University, Egypt, for his help in creating a phylogenetic tree. |
||||||||||||||||||||||||||||||||||||||||||||
|
|
||||||||||||||||||||||||||||||||||||||||||||
|
Conflict of Interests |
||||||||||||||||||||||||||||||||||||||||||||
|
The author declares no conflicts of interest associated with this manuscript. |
||||||||||||||||||||||||||||||||||||||||||||
|
|
||||||||||||||||||||||||||||||||||||||||||||
|
References |
||||||||||||||||||||||||||||||||||||||||||||
|
|
||||||||||||||||||||||||||||||||||||||||||||
|
Abdel-Baki, A.S., Abdel-Haleem, H.M., Al-Quraishy, S., 2012. A new Sarcocystis species (Apicomplexa: Sarcocystidae) from the Rock Gecko Bunopus tuberculatus in SaudiArabia. Journal of Parasitology 98, 951–953. Abdel-Ghaffar, F., Al Johany, A.M., 2002. A light and electron microscope study of Sarcocystis mitrani (sp. Nov.) infecting the skink Scincus mitranus in the central region of Saudi Arabia. Parasitology Research 88,102–106. Abdel-Ghaffar, F., Bashtar, A.R, Ashour, MB, Sakran, T.H., 1990a. Life cycle of Sarcocystis gongyli Trinci 1911 in the shink Chalcides ocellatus and the snake Spalerosophis diadema. Parasitology Research 76, 444–450. Abdel-Ghaffar, F., Bashtar, A.R, El-Sayed, M., 1990b. Electron microscopic studies on Sarcocystis infection in sheep in Upper Egypt. Bulletin of the faculty of science. Cairo University 58, 33–49. Abdel-Ghaffar, F., Mehlhorn, H., Bashtar, A.R, Al-Rasheid, K., Sakran, T., El-Fayoumi, H., 2009. Life cycle of Sarcocystis camelicanis infecting the camel (Camelus dromedaries) and the dog (Canis familiaris), light and electron microscopic study. Parasitology Research 106, 189–195. Al Quraishy, S., Morsy, K., Bashtar, A.R., Abdel-Ghaffar, F., Mehlhorn, H., 2014. Sarcocystis arieticanis (Apicomplexa: Sarcocystidae) infecting the heart muscles of the domestic sheep, Ovis aries (Artiodactyla: Bovidae), from K. S. A. on the basis of light and electron microscopic data. Parasitology Research 113, 3823–3831. Balbo, T., Rossi, L., Lanfranchi, P., Meneguz, P.G., Meneghi, D. De., Canese, M.G., 1988 Experimental transmission of a sarcosporidian from alpine ibex to domestic sheep and goats. Parasitologica 30, 241–247. Bashtar, A.R, Abdel-Ghaffar, F., EL-Assal, F., Sakran, T.H., 1990. Incidence and prevalence of Sarcocystis infecting sheep at Beni Suef province and dog as a final host. Bulletin of the faculty of science. Cairo University 58, 53–70. Bashtar, A.R, Sakran, T., Shazly, H., EL-Ganiny, M.A.S., 1992. Gamogony and sporogony of Sarcocystis fusiformis infecting the water buffalo in the small intestine of cats. Bulletin of the faculty of science. Cairo University 60, 113–126. Bittencourt, M.V., Meneses, I.D.S., Andrade, M.R., Jesus, R.F., Araújo, F.R., Condim, L.F.P., 2016. Sarcocystis spp. in sheep and goats: frequency of infection and species identification by morphological, ultrastructural, and molecular tests in Bahia, Brazil. Parasitology Research 115, 1683–1689. Blasco-Costa, I., Cutmore, S.C., Miller, T.L., Nolan, M.J., 2016. Molecular approaches to trematode systematics: ‘best practice’ and implications for future study. Systematic Parasitology 93, 295–306. Dubey, J.P., Lindsay, D.S., Speer, C.A., Fayer, R., Livingstone, C.W., 1988. Sarcocystis arieticanis and other Sarcocystis species in sheep in the United States. Journal of Parasitology 74, 1033–1038. Dubey, J.P., Speer, C.A., Fayer, R., 1989. Sarcocystosis of Animals and Man. CRC Press, Inc., Florida, Tropical Animal Health and Production 215, 62–78. Dubey, J.P., Rosenthal, B.M., 2013. Sarcocystis capracanis associated encephalitis in sheep. Veterinary Parasitology 197, 407–408. Dubey, J.P., Calero-Bernal, R., Rosenthal, B.M., Speer, C.A., Fayer, R., 2016. Sarcocystosis of animals and humans, 2nd edn. Boca Raton, Florida: CRC Press. pp. 195–214, 217–234, 243–248, 273–275 Edgar, R.C., 2004. MUSCLE: multiple sequence alignment with high accuracy and high throughput. Nucleic Acids Research 32, 1792–1797. El-Morsey, A., Abdo, W., Sultan, Kh., Elhawary, N.M., Abou Zaid, A.A., 2019. Ultrastructural and molecular identification of the sarcocysts of Sarcocystis tenella and Sarcocystis arieticanis infecting domestic sheep (Ovis aries) from Egypt. Acta Parasitologica 64, 501–513. Fayer, R., 2004. Sarcocystis spp. in human infections. Clinical Microbiology Reviews 17, 894–902. Formisano, P., Aldridge, B., Alony, Y., Beekhuis, L., Davies, E., Del Pozo, J., Dunn, K., English, K., Morrison, L., Sargison, N., Seguino, A., Summers, B.A., Wilson, D., Milne, E., Beard, P.M., 2013. Identification of Sarcocystis capracanis in cerebrospinal fluid from sheep with neurological disease. Veterinary Parasitology 193, 252–255. Haziroglu, R., Guvenc, T., Tunca, R., 2003. Electron microscopical studies on cysts of Sarcocystis arieticanis within cardiac muscle of naturally infected sheep. Parasitology Research 89, 23–25. Heydorn, A.O., 1985. Zur Entwicklung von Sarcocystis arieticanis n.sp. Berliner und Muenchener Tieraerztliche Wochenschrift 98, 231–241. Heydorn, A.O., Mehlhorn, H., 1987. Fine structure of Sarcocystis arieticanis Heydorn, 1985 in its intermediate and final hosts (sheep and dog). Zentralblatt fur Bakteriologie, Mikrobiologie und Hygiene A 264, 353–362. Hu, J.J., Huang, S., Wen, T., Esch, G.W, Liang, Y., Li, H.L., 2017. Sarcocystis spp. in domestic sheep in Kunming City, China: prevalence, morphology, and molecular characteristics. Parasite 24,30. Hu, J.J., Liu, T.T., Liu, Q., Esch, G.W., Chen, J.Q., Huang, S., Wen, T., 2016. Prevalence, morphology, and molecular characteristics of Sarcocystis spp. in domestic goats (Capra hircus) from Kunming, China. Parasitology Research 115, 3973–3981. Hussein, N.M., Hassan, A.A., Abd- Ella, O.H., 2018. Morphological, ultrastructural, and molecular characterization of Sarcocystis tenella from heep in Qena Governorate, Upper Egypt. Egyptian Academic Journal of Biolog Science 10, 11 – 19. Jehle, C., Dinkel, A., Sander, A., Morent, M., Romig, T., Luc, P.V., De, T.V., Thai, V.V., Mackenstedt, U., 2009. Diagnosis of Sarcocystis spp. in cattle (Bos taurus) and water buffalo (Bubalus bubalis) in Northern Vietnam. Veterinary Parasitolology 166, 314–320. Kumar, S., Stecher, G., Tamura, K., 2016. MEGA7: molecular evolutionary genetics analysis version 7.0 for bigger datasets. Molecular Biology and Evolution 33, 1870–1874. Lam, K.K.H., Watkins, K.L., Chan, C.W., 1999. First report of equine protozoal myeloencephalitis in Hong Kong. Equine Veterinary Education 11, 54–56. Levine, N.D., 1985. Veterinary Protozoology (1st edition). Lowa State, University press, Ames. Mahran, O.M., 2009. Sarcocystis infection in sheep and goats slaughtered in Shalatin Abattoir, Red Sea Governorate, Egypt. Assiut Veterinary Medical Journal 55, 341–55. Mandour, A.M., Rabie, S.A, Mohammed, N.I, Hussein, N.M., 2011. On the presence of Sarcocystis miescheri sp. nov. in camels of Qena Governorate. Egyptian Academic Journal of Biological Science. Medical Entomology and Parasitology 3, 1–7. Mehlhorn , H., Heydorn, A.O., 1979. Electron microscopical study on gamogony of Sarcocystis suihominis in human tissue cultures. Zeitschrift Parasitenkunde 58,97–113. Mehlhorn, H., Abdel-Ghaffar, F., Bashtar, A.R., Morsy, K., Saleh, A., 2014. Sarcocystis arieticanis (Apicomplexa: Sarcocystidae) infecting the heart muscles of the domestic sheep, Ovis aries (Artiodactyla: Bovidae), from K. S. A. on the basis of light and electron microscopic data. Parasitology Research 113, 3823–3831. Morsy, K., Saleh, A., Al-ghamdi, A., Abdel-Ghaffar, F., Al-Rasheid, K.h., Bashtar, A.R., Al-Quraishy, S., Mehlhorn, H., 2011. Prevalence pattern and biology of Sarcocystis capracanis in the Egyptian goats: A light and ultrastructural study. Veterinary Parasitology 181, 75–82. Nei, M., Kumar, S., 2000. Molecular evolution and phylogenetics. Oxford University Press, New York. Odening, K., Stolte, M., Walter, G., Bockhardt, I., 1995. Cyst wall ultrastructure of two Sarcocystis spp. from European mouflon (Ovis ammon musimon) in Germany compared with domestic sheep. Journal of Wildlife Diseases 31, 550–554. Odening, K., Stolte, M., Bockhardt, I., 1996. Sarcocysts in exotic Caprinae (Bovidae) from zoological gardens. Acta Parasitologica 41, 67–75. Olias, P., Oliasa, L., Michael, L., Mehlhorn, H., Grubera, A., 2010. Sarcocystis calchasi is distinct to Sarcocystis columbae sp. nov. from wood pigeon (Columba palumbus) and Sarcocystis sp. from the sparrow hawk (Accipiter nisus). Veterinary Parasitolology 171, 7–14. Saito, M., Shibata, Y., Kubo, M., Itagaki, H., 1997. Sarcocystis mihoensis n. sp. from sheep in Japan. Journal of Veterinary Medical Science 59, 103–106. Tenter, A.M., 1995. Current research on Sarcocystis species of domestic animals. International Journal of Parasitology 25, 1311–1130 Wang, G., Wei, T., Wang, X., Li, W., Zhang, P., Dong, M., Xiao, H., 1988. The morphology and life cycle of Sarcocystis microps n. sp. in sheep of Qinghai in China. China Veterinary Technology 6, 9–11. Xiang, Z., Rosenthal, R., Yongshu, H., Wang, W., Wang, H., Song, J., Shen, P., Li, M., Yang, Z., 2010. Sarcocystis tupaia, sp. nov., a new parasite species employing treeshrews (Tupaiidae, Tupaia belangeri chinensis) as natural intermediate hosts. Parasitology International 59, 128–132. Yang, Z.Q., Li, Q.Q., Zuo, Y.X., Chen, X.W., Chen, Y.J., Nie, L., Wei, C.G, Zen, J.S., Attwood, S.W., Zhang, Z., Zhang, Y.P., 2002. Characterization of Sarcocystis species in domestic animals using a PCR-RFLP analysis of variation in the 18S rRNA gene: a cost-effective and simple technique for routine species identification. Experimental Parasitology 102, 212– 217. |
||||||||||||||||||||||||||||||||||||||||||||
|
|